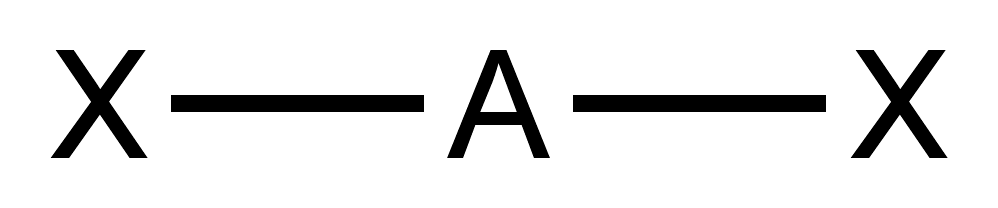|
Silylgermane
Silylgermane is an inorganic compound with the chemical formula . It is a colorless gas with an unpleasant odor. It is unstable in air. It is very flammable, very toxic and corrosive. It reacts with alkali liberating hydrogen. History Silylgermane was prepared in 1962 by Alan MacDiarmid and his coworkers by a silent electric discharge through an equimolar mix of silane and germane gases for a total of 28 hours. This method produces other hydrides of silicon and germanium as well. Preparation One of the most common methods of preparation of silylgermane is the reaction of germane () and chlorosilane () in the presence of a catalyst. Another method is the reaction of germane and silicon tetrachloride in the presence of a reducing agent. Silylgermane can also be produced by the reaction between hydrochloric acid and magnesium or calcium germanides-silicides, and by the reaction between hydrofluoric acid and a mixture of silicon monoxide and germanium monoxide. The synthesis of silyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group 14 Hydride
Group 14 hydrides are chemical compounds composed of hydrogen atoms and carbon group, group 14 atoms (the elements of group 14 are carbon, silicon, germanium, tin, lead and flerovium). Tetrahydrides The tetrahydride series has the chemical formula , with X representing any of the carbon family. Methane is commonly the result of the decomposition of organic matter and is a greenhouse gas. The other hydrides are generally unstable, poisonous metal hydrides. They take on a pyramidal structure, and as such are not polar molecules like the other p-block hydrides. Unlike other light hydrides such as ammonia, water and hydrogen fluoride, methane does not exhibit any anomalous effects attributed to hydrogen bonding, and so its properties conform well to the prevailing trend of heavier group 14 hydrides. Hexahydrides This series has the chemical formula . Ethane is commonly found alongside methane in natural gas. The other hydrides of the chemical formula are less stable than the cor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethane
Ethane ( , ) is a naturally occurring Organic compound, organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is List of purification methods in chemistry, isolated on an industrial scale from natural gas and as a petrochemical by-product of oil refinery, petroleum refining. Its chief use is as feedstock for ethylene production. The ethyl group is formally, although rarely practically, derived from ethane. History Ethane was first synthesised in 1834 by Michael Faraday, applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further. The process is now called Kolbe electrolysis: : acetate, CH3COO− → CH3• + carbon dioxide, CO2 + electron, e− : CH3• + •CH3 → C2H6 During the period 1847–1849, in an effort to vindicate the radical theory of organic chemistry, Hermann Kolbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Tetrachloride
Silicon tetrachloride or tetrachlorosilane is the inorganic compound with the formula SiCl4. It is a colorless volatile liquid that fumes in air. It is used to produce high purity silicon and silica for commercial applications. It is a part of the chlorosilane family. Preparation Silicon tetrachloride is prepared by the chlorination of various silicon compounds such as ferrosilicon, silicon carbide, or mixtures of silicon dioxide and carbon. The ferrosilicon route is most common. In the laboratory, can be prepared by treating silicon with chlorine at : : It was first prepared by Jöns Jakob Berzelius in 1823. Brine can be contaminated with silica when the production of chlorine is a byproduct of a metal refining process from metal chloride ore. In rare occurrences, the silicon dioxide in silica is converted to silicon tetrachloride when the contaminated brine is electrolyzed. Reactions Hydrolysis and related reactions Like other chlorosilanes or silanes, silicon tetrachl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VSEPR Theory
Valence shell electron pair repulsion (VSEPR) theory ( , ) is a conceptual model, model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Sydney Nyholm, Ronald Nyholm. The premise of VSEPR is that the valence electron pairs surrounding an atom tend to repel each other. The greater the repulsion, the higher in energy (less stable) the molecule is. Therefore, the VSEPR-predicted molecular geometry of a molecule is the one that has as little of this repulsion as possible. Gillespie has emphasized that the electron-electron repulsion due to the Pauli exclusion principle is more important in determining molecular geometry than the electrostatic repulsion. The insights of VSEPR theory are derived from topological analysis of the electron density of molecules. Such quantum chemical topology (QCT) metho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silyl
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. Silylations are core methods for production of organosilicon chemistry. Silanization, while similar to silylation, usually refers to attachment of silyl groups to solids. Silyl groups are commonly used for: alcohol protection, enolate trapping, gas chromatography, electron-impact mass spectrometry (EI-MS), and coordinating with metal complexes. Protection Chemistry Protection Silylation is often used to protect alcohols, as well as amines, carboxylic acids, and terminal alkynes. The products after silylation, namely silyl ethers and silyl amines, are resilient toward basic conditions. Protection is typically done by reacting the functional group with a silyl halide by an SN2 reaction mechanism, typically in the presence of base. The protection mechanism begins with the base deprotonating the alcohol group. Next, the deprotonated alcohol group attacks the silyl atom of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanium
Germanium is a chemical element; it has Symbol (chemistry), symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid or a nonmetal in the carbon group that is chemically similar to silicon. Like silicon, germanium naturally Chemical reaction, reacts and forms complexes with oxygen in nature. Because it seldom appears in high concentration, germanium was found comparatively late in the Timeline of chemical element discoveries, discovery of the elements. Germanium ranks 50th Abundance of elements in Earth's crust, in abundance of the elements in the Earth's crust. In 1869, Dmitri Mendeleev Mendeleev's predicted elements, predicted its existence and some of its Chemical property, properties from its position on his periodic table, and called the element ekasilicon. On February 6, 1886, Clemens Winkler at Freiberg University found the new element, along with silver and sulfur, in the mineral argyrodite. Winkle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Silicon is a significant element that is essential for several physiological and metabolic processes in plants. Silicon is widely regarded as the predominant semiconductor material due to its versatile applications in various electrical devices such as transistors, solar cells, integrated circuits, and others. These may be due to its significant band gap, expansive optical transmission range, extensive absorption spectrum, surface roughening, and effective anti-reflection coating. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanium Monoxide
Germanium monoxide (chemical formula GeO) is a chemical compound of germanium and oxygen. It can be prepared as a yellow sublimate at 1000 °C by reacting GeO2 with Ge metal. The yellow sublimate turns brown on heating to 650 °C. GeO is not well characterised. It is amphoteric, dissolving in acids to form germanium(II) salts and in alkali to form "trihydroxogermanates" or "germanites" containing the Ge(OH)3− ion.Egon Wiberg, Arnold Frederick Holleman (2001) ''Inorganic Chemistry'', Elsevier Chemistry Germanium oxide decomposes to Ge and GeO2.Shriver and Atkins. Inorganic Chemistry (5th Edition). W. H. Freeman and Company, New York, 2010, pp 365. See also *Germanium dioxide Germanium dioxide, also called germanium(IV) oxide, germania, and salt of germanium, is an inorganic compound with the chemical formula Ge O2. It is the main commercial source of germanium. It also forms as a passivation layer on pure germaniu ... References Germanium(II) compounds O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Monoxide
Silicon monoxide is the chemical compound with the formula SiO where silicon is present in the oxidation state +2. In the vapour phase, it is a diatomic molecule. It has been detected in stellar objects and has been described as the most common oxide of silicon in the universe.Peter Jutzi and Ulrich Schubert (2003) ''Silicon chemistry: from the atom to extended systems''. Wiley-VCH . Solid form When SiO gas is cooled rapidly, it condenses to form a brown/black polymeric glassy material, (SiO)''n'', which is available commercially and used to deposit films of SiO. Glassy (SiO)''n'' is air and moisture sensitive. Oxidation Its surface readily oxidizes in air at room temperature, giving an SiO2 surface layer that protects the material from further oxidation. However, (SiO)''n'' irreversibly disproportionates into SiO2 and Si in a few hours between 400 °C and 800 °C and very rapidly between 1,000 °C and 1,440 °C, although the reaction does not go to complet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling point near room temperature. It is used to make most organofluorine compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicide
A silicide is a type of chemical compound that combines silicon and a usually more electropositive element. Silicon is more electropositive than carbon. In terms of their physical properties, silicides are structurally closer to borides than to carbides. Because of size differences however silicides are not isostructural with borides and carbides. Bonds in silicides range from conductive metal-like structures to covalent or ionic. Silicides of all non-transition metals have been described except beryllium. Silicides are used in interconnects. Structure Silicon atoms in silicides can have many possible organizations: *Isolated silicon atoms: electrically conductive (or semiconductive) CrSi, MnSi, FeSi, CoSi, , , , , , *Si2 pairs: , hafnium and thorium silicides *Si4 tetrahedra: KSi, RbSi, CsSi *Si''n'' chains: USi, , CaSi, SrSi, YSi *Planar hexagonal graphite-like Si layers: β-USi2, silicides of other lanthanoids and actinoids *Corrugated hexagonal Si layers: C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanide
A germanide is any binary compound of germanium and a more electropositive element. The composition of most germanides is analogous to that of the corresponding silicides and does not follow formal valence rules. The germanides of alkali and alkaline earth metals, are readily decomposed by water and acids to give germanium hydrides; most germanides of the transition metal In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...s resist the action of acids and alkalies. The main method of producing germanides is the melting or sintering of the components. The IUPAC Red Book uses the name tetragermide for compounds containing and instead uses the term germanide (or trihydridogermanate(1-)) for the anion. Examples * Copper germanide References {{Monatomic anion compounds * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |