|
Quinagolide
Quinagolide (, ), sold under the brand name Norprolac, is a binding selectivity, selective dopamine receptor, dopamine D2 receptor, D2 receptor agonist which is used to manage hyperprolactinemia. It has also been found to be effective in the treatment of breast pain. It is used in the UK, but it is not available in US. Chemistry Quinagolide is a racemate composed of the following two enantiomers: Synthesis Laboratory synthesis The first synthesis of quinagolide was disclosed in patents filed by Sandoz. : A sequence of nine steps is required to transform the starting material 5-methoxy-2-tetralone (1) into the octahydrobenzo[g]quinoline ring system with the correct stereochemistry required. This intermediate (11) is then converted in another five steps to the drug. Transformation of the ester (13) into the amine (15) is accomplished by a Curtius rearrangement in which an Hydrazide#Acyl_hydrazides, acyl hydrazide is treated with nitrosyl chloride. : Manufacture The laboratory rout ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperprolactinemia
Hyperprolactinaemia (also spelled hyperprolactinemia) is a condition characterized by abnormally high levels of prolactin in the blood. In women, normal prolactin levels average to about 13 ng/mL, while in men, they average 5 ng/mL. The upper normal limit of serum prolactin is typically between 15 and 25 ng/mL for both genders. Levels exceeding this range indicate hyperprolactinemia. Prolactin (PRL) is a peptide hormone produced by lactotroph cells in the anterior pituitary gland. It plays a vital role in lactation and breast development. Hyperprolactinemia, characterized by abnormally high levels of prolactin, may cause galactorrhea (production and spontaneous flow of breast milk), infertility, and menstrual disruptions in women. In men, it can lead to hypogonadism, infertility and erectile dysfunction. Prolactin is crucial for milk production during pregnancy and lactation. Together with estrogen, progesterone, insulin-like growth factor-1 (IGF-1), and horm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
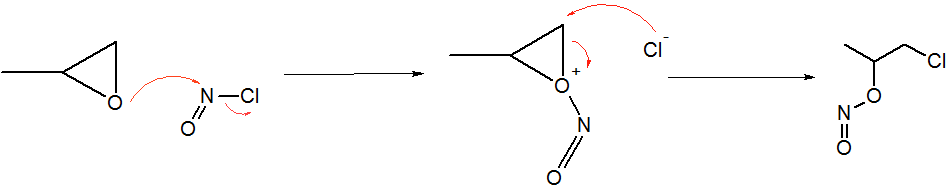
Quinagolide Synthesis (part 1)
Quinagolide (, ), sold under the brand name Norprolac, is a selective dopamine D2 receptor agonist which is used to manage hyperprolactinemia. It has also been found to be effective in the treatment of breast pain. It is used in the UK, but it is not available in US. Chemistry Quinagolide is a racemate composed of the following two enantiomers: Synthesis Laboratory synthesis The first synthesis of quinagolide was disclosed in patents filed by Sandoz. : A sequence of nine steps is required to transform the starting material 5-methoxy-2-tetralone (1) into the octahydrobenzo uinoline ring system with the correct stereochemistry required. This intermediate (11) is then converted in another five steps to the drug. Transformation of the ester (13) into the amine (15) is accomplished by a Curtius rearrangement in which an acyl hydrazide is treated with nitrosyl chloride. : Manufacture The laboratory route was not practical for the synthesis of quinagoline on a large scale. Therefore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosyl Chloride
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound. Structure and synthesis The molecule is bent. A double bond exists between N and O (distance = 1.16 Å) and a single bond between N and Cl (distance = 1.96 Å). The O=N–Cl angle is 113°. Production Nitrosyl chloride can be produced in many ways. * Combining nitrosylsulfuric acid and HCl affords the compound. This method is used industrially. :HCl + NOHSO4 → H2SO4 + NOCl * A more convenient laboratory method involves the (reversible) dehydration of nitrous acid by HCl : HNO2 + HCl → H2O + NOCl * By the direct combination of chlorine and nitric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-iodopropane
''n''-Propyl iodide (also 1-propyl iodide or 1-iodopropane) is a colorless, flammable chemical compound. It has the chemical formula C3H7I and is prepared by heating ''n''-propyl alcohol with iodine and phosphorus.''Merck Index ''The Merck Index'' is an encyclopedia of chemical substance, chemicals, pharmaceutical drug, drugs and biomolecule, biologicals with over 10,000 monographs on single substances or groups of related chemical compound, compounds published online ...'', 9th ed., monograph 7651 References Iodoalkanes Propyl compounds {{Organohalide-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylated
Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion (carbanion). The formal "alkyl anion" attacks an electrophile, forming a new covalent bond between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fischer Esterification
Fischer is a German occupational surname, meaning fisherman. The name Fischer is the fourth most common German surname. The English version is Fisher. People with the surname A * Abraham Fischer (1850–1913) South African public official * Adam Fischer (sculptor) (1888–1968), Danish sculptor * Ádám Fischer (born 1949), Hungarian conductor * Adolf Fischer (officer) (1893–1947), German Nazi general executed for war crimes * Adolph Fischer (1858–1887) German-American anarchist * Alfred Fischer (architect) (1881–1950), German architect * Alfred Fischer (judge) (1919–2004), German judge * Andrew Andika Fischer (born 1987), Indonesian actor * Angeline Fuller Fischer (1841–1925), American writer * Annie Fischer (1914–1995), Hungarian pianist * Andrea Fischer (born 1960), German politician * Andrea Fischer (scientist), (born 1973), Austrian glaciologist * Anton Fischer (bobsleigh), German bobsledder * Artur Fischer (1919–2016), German inventor (fischertec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodium borohydride is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis. The compound was discovered in the 1940s by H. I. Schlesinger, who led a team seeking volatile uranium compounds.Hermann I Schlesinger and Herbert C Brown (1945)Preparation of alkali metal compounds. US Patent 2461661. Granted on 1949-02-15; expired on 1966-02-15. Results of this wartime research were declassified and published in 1953. Properties The compound is soluble in alcohols, certain ethers, and water, although it slowly hydrolyzes. Sodium borohydride is an odorless white to gray-white microcrystalline powder that often forms lumps. It can be purified by recrystallization from warm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions. Distinction is sometimes made between aldimines and ketimines, derived from aldehydes and ketones, respectively. Structure In imines the five core atoms (C2C=NX, ketimine; and C(H)C=NX, aldimine; X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually double-bonded carbon and the nitrogen atoms. The C=N distance is 1.29–1.31 Å for nonconjugated imines and 1.35 Å for conjugated imines. By contrast, C−N distances in amines and nitriles are 1.47 and 1.16 Å respectively. Rotation about the C=N bond is slow. Using NMR spectroscopy, both E–Z notation, ''E'' and ''Z'' isomers of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Birch Reduction
The Birch reduction or Metal-Ammonia reduction is an organic reaction that is used to convert arenes to Cyclohexa-1,4-diene, 1,4-cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch (organic chemist), Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent (traditionally liquid ammonia) with an alkali metal (traditionally sodium) and a Hydron (chemistry), proton source (traditionally an Alcohol (chemistry), alcohol). Unlike catalytic hydrogenation, Birch reduction does not reduce the aromatic ring all the way to a cyclohexane. An example is the reduction of naphthalene in ammonia and ethanol: Reaction mechanism and regioselectivity A solution of sodium in liquid ammonia consists of the intensely blue electride salt [Na(NH3)x]+ e−. The solvated electrons add to the aromatic ring to give a radical ion, radical anion, which then abstracts a proton from the alcohol. The process then repeats at either the Arene substit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |