|
Prokaryotic DNA Replication
Prokaryotic DNA Replication is the process by which a prokaryote duplicates its DNA into another copy that is passed on to daughter cells. Although it is often studied in the model organism '' E. coli'', other bacteria show many similarities. Replication is bi-directional and originates at a single origin of replication (OriC). It consists of three steps: Initiation, elongation, and termination. Initiation All cells must finish DNA replication before they can proceed for cell division. Media conditions that support fast growth in bacteria also couples with shorter inter-initiation time in them, i.e. the doubling time in fast growing cells is less as compared to the slow growth. In other words, it is possible that in fast growth conditions the grandmother cells starts replicating its DNA for grand daughter cell. For the same reason, the initiation of DNA replication is highly regulated. Bacterial origins regulate orisome assembly, a nuclei-protein complex assembled on the or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prokaryotic
A prokaryote () is a single-celled organism that lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Greek πρό (, 'before') and κάρυον (, 'nut' or 'kernel').Campbell, N. "Biology:Concepts & Connections". Pearson Education. San Francisco: 2003. In the two-empire system arising from the work of Édouard Chatton, prokaryotes were classified within the empire Prokaryota. But in the three-domain system, based upon molecular analysis, prokaryotes are divided into two domains: ''Bacteria'' (formerly Eubacteria) and ''Archaea'' (formerly Archaebacteria). Organisms with nuclei are placed in a third domain, Eukaryota. In the study of the origins of life, prokaryotes are thought to have arisen before eukaryotes. Besides the absence of a nucleus, prokaryotes also lack mitochondria, or most of the other membrane-bound organelles that characterize the eukaryotic cell. It was once thought that prokaryotic cellular components within the cytopla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SeqA Protein Domain
In molecular biology the SeqA protein is found in bacteria and archaea. The function of this protein is highly important in DNA replication. The protein negatively regulates the initiation of DNA replication at the origin of replication, in ''Escherichia coli'', OriC. Additionally the protein plays a further role in sequestration. The importance of this protein is vital, without its help in DNA replication, cell division and other crucial processes could not occur. This protein domain is thought to be part of a much larger protein complex which includes other proteins such as SeqB. Function DNA replication is an energy consuming process and hence in bacteria the process only occurs at a specific checkpoint in the cell cycle. The binding of SeqA protein to hemimethylated GATC sequences is important in the negative modulation of chromosomal initiation at oriC, and in the formation of SeqA foci necessary for ''Escherichia coli'' chromosome segregation. SeqA tetramers are able to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
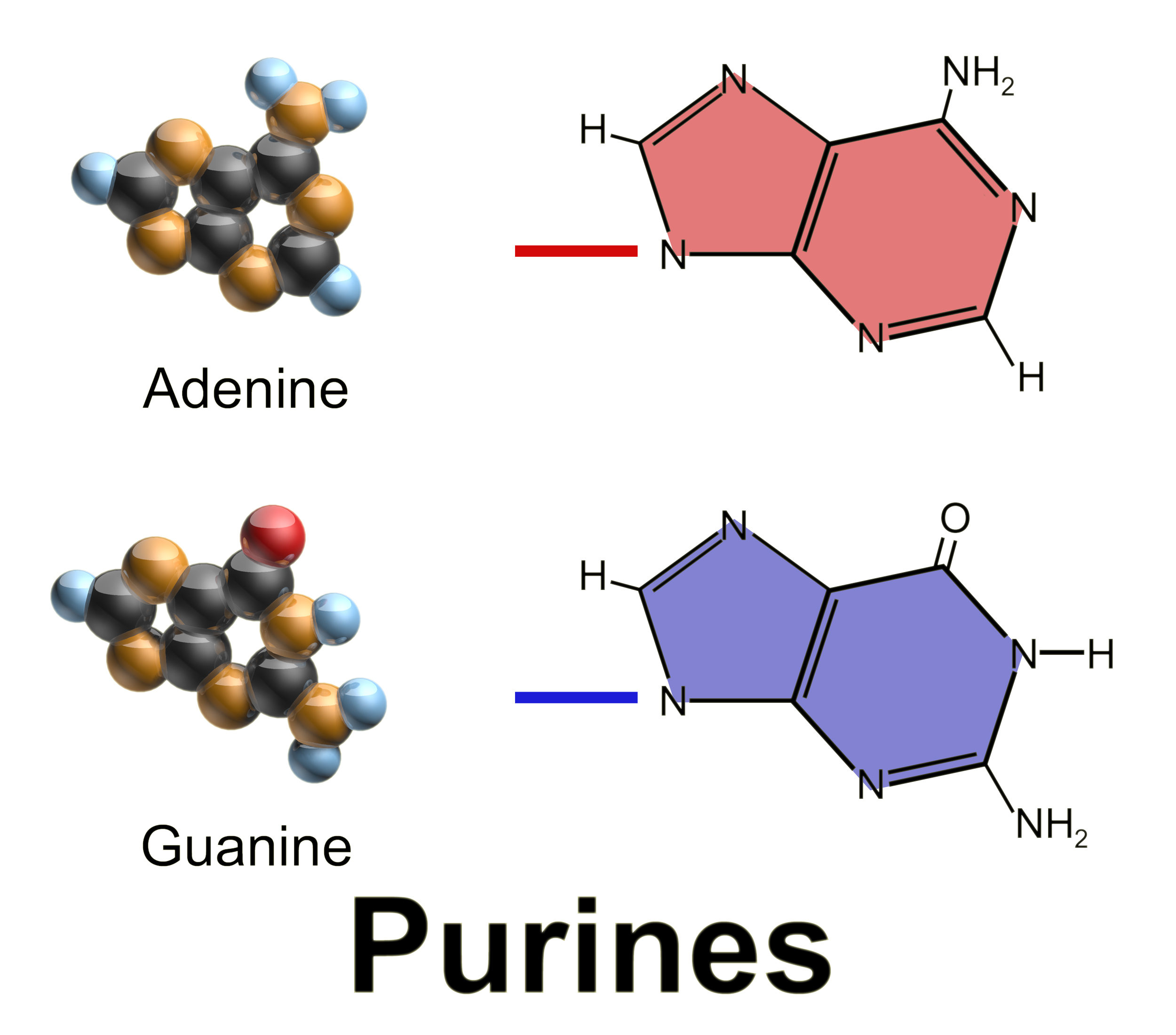
Purine
Purine is a heterocyclic aromatic organic compound that consists of two rings ( pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature. Dietary sources Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. In general, plant-based diets are low in purines. High-purine plants and algae include some legumes (lentils and black eye peas) and spirulina. Examples of high-purine sources include: sweetbreads, anchovies, sardines, liver, beef kidneys, brains, meat extracts (e.g., Oxo, Bovril), herring, mackerel, scallops, game meats, yeast (beer, yeast extract, nutritional yeast) and gravy. A moderate amount of purine is also contained in red meat, beef, pork, poultry, fish and seafood, asparagus, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many industrially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Minor Groove
Minor may refer to: * Minor (law), a person under the age of certain legal activities. ** A person who has not reached the age of majority * Academic minor, a secondary field of study in undergraduate education Music theory * Minor chord ** Barbershop seventh chord or minor seventh chord * Minor interval * Minor key *Minor scale Mathematics * Minor (graph theory), the relation of one graph to another given certain conditions * Minor (linear algebra), the determinant of a certain submatrix People * Charles Minor (1835–1903), American college administrator * Charles A. Minor (21st-century), Liberian diplomat * Dan Minor (1909–1982), American jazz trombonist * Dave Minor (1922–1998), American basketball player * James T. Minor, US academic administrator and sociologist * Jerry Minor (born 1969), American actor, comedian and writer * Kyle Minor (born 1976), American writer * Mike Minor (actor) (born 1940), American actor * Mike Minor (baseball) (born 1987), American ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Major Groove
Major (commandant in certain jurisdictions) is a military rank of commissioned officer status, with corresponding ranks existing in many military forces throughout the world. When used unhyphenated and in conjunction with no other indicators, major is one rank above captain, and one rank below lieutenant colonel. It is considered the most junior of the field officer ranks. Background Majors are typically assigned as specialised executive or operations officers for battalion-sized units of 300 to 1,200 soldiers while in some nations, like Germany, majors are often in command of a company. When used in hyphenated or combined fashion, the term can also imply seniority at other levels of rank, including ''general-major'' or ''major general'', denoting a low-level general officer, and ''sergeant major'', denoting the most senior non-commissioned officer (NCO) of a military unit. The term ''major'' can also be used with a hyphen to denote the leader of a military band such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules. Named after Dutch physicist Johannes Diderik van der Waals, the van der Waals force plays a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. It also underlies many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media. If no other force is present, the distance between atoms at which the force becomes repulsive rather than attractive as the atoms approach one another is called the van der Waals contact distance; this phenomen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrophosphate
In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P–O–P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate (Na2H2P2O7) and tetrasodium pyrophosphate (Na4P2O7), among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. The pyrophosphate bond is also sometimes referred to as a phosphoanhydride bond, a naming convention which emphasizes the loss of water that occurs when two phosphates form a new P–O–P bond, and which mirrors the nomenclature for anhydrides of carboxylic acids. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate, as for dimethylallyl pyrophosphate. This bond is also referred to as a high- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one or two protons gives the dihydrogen phosphate ion and the hydrogen phosphate ion ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, . The term also refers to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and ''electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''cationoi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an atom of a given element is determined by the number of hydrogen atoms that it combines with. In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of 2; and in hydrogen chloride, chlorine has a valence of 1. Chlorine, as it has a valence of one, can be substituted for hydrogen. Phosphorus has a valence of 5 in phosphorus pentachloride, . Valence diagrams of a compound represent the connectivity of the elements, with lines drawn between two elements, sometimes called bonds, representing a saturated valency for each element. The two tables below show some examples of different compounds, their valence diagrams, and the valences for each element of the compound. Modern definition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribonucleotide
In biochemistry, a ribonucleotide is a nucleotide containing ribose as its pentose component. It is considered a molecular precursor of nucleic acids. Nucleotides are the basic building blocks of DNA and RNA. Ribonucleotides themselves are basic monomeric building blocks for RNA. Deoxyribonucleotides, formed by reducing ribonucleotides with the enzyme ribonucleotide reductase (RNR), are essential building blocks for DNA. There are several differences between DNA deoxyribonucleotides and RNA ribonucleotides. Successive nucleotides are linked together via phosphodiester bonds. Ribonucleotides are also utilized in other cellular functions. These special monomers are utilized in both cell regulation and cell signaling as seen in adenosine-monophosphate ( AMP). Furthermore, ribonucleotides can be converted to adenosine triphosphate (ATP), the energy currency in organisms. Ribonucleotides can be converted to cyclic adenosine monophosphate (cyclic AMP) to regulate hormones in organisms as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |