|
Oxidizers
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an Redox, oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perchlorate
A perchlorate is a chemical compound containing the perchlorate ion, . The majority of perchlorates are commercially produced salts. They are mainly used as oxidizers for pyrotechnic devices and to control static electricity in food packaging. Perchlorate contamination in food, water, and other parts of the environment has been studied in the U.S. because of harmful effects on human health. Perchlorate ions are somewhat toxic to the thyroid gland. Most perchlorates are colorless solids that are soluble in water. Four perchlorates are of primary commercial interest: ammonium perchlorate , perchloric acid , potassium perchlorate and sodium perchlorate . Perchlorate is the anion resulting from the dissociation of perchloric acid and its salts upon their dissolution in water. Many perchlorate salts are soluble in non-aqueous solutions. Production Perchlorate salts are produced industrially by the oxidation of aqueous solutions of sodium chlorate by electrolysis. This method i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
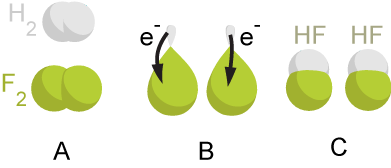
Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ). Examples of substances that are commonly reducing agents include the Earth metals, formic acid, oxalic acid, and sulfite compounds. In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/ donates electrons", that "i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidize
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magic Blue
Tris(4-bromophenyl)ammoniumyl hexachloroantimonate is the organic compound with the formula 4-BrC6H4)3NbCl6. Commonly known as magic blue, it is the hexachloroantimonate salt of an amine radical cation. It is a blue solid that reacts with many solvents but is soluble in acetonitrile. The compound is a popular oxidizing agent in organic and organometallic chemistry, with a reduction potential of 0.67 V versus ferrocene/ferrocenium ( acetonitrile solution) or 0.70 V versus ferrocene/ferrocenium (dichloromethane Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible wit ... solution). The structure of the cation consists of a three-bladed propeller structure with a planar amine. It is nearly identical to the parent triphenylamine. The weakly coordinating anion is , which is octahed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reviews
''Chemical Reviews'' is peer-reviewed scientific journal published twice per month by the American Chemical Society. It publishes review articles on all aspects of chemistry. It was established in 1924 by William Albert Noyes ( University of Illinois). the editor-in-chief is Sharon Hammes-Schiffer. Abstracting and indexing The journal is abstracted and indexed in Chemical Abstracts Service, CAB International, EBSCOhost, ProQuest, PubMed, Scopus, and the Science Citation Index. According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as ... of 60.622. See also * Accounts of Chemical Research References External links * American Chemical Society academic journals Review journals Monthly jo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Electrode Potential (data Page)
The data values of standard electrode potentials (''E''°) are given in the table below, in volts relative to the standard hydrogen electrode, and are for the following conditions: * A temperature of . * An effective concentration of 1 mol/L for each aqueous species or a species in a mercury amalgam (an alloy of mercury with another metal). * A partial pressure of 101.325 kPa (absolute) (1 atm, 1.01325 bar) for each gaseous reagent. This pressure is used because most literature data are still given for this value (1 atm) rather than for the current standard of 100 kPa (1 bar) presently considered in the standard state. * An activity of unity for each pure solid, pure liquid, or for water (solvent). The relation in electrode potential of metals in saltwater (as electrolyte) is given in the '' galvanic series''. * Although many of the half cells are written for multiple-electron transfers, the tabulated potentials are for a single-electron transfer. All of the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmium Tetroxide
Osmium tetroxide (also osmium(VIII) oxide) is the chemical compound with the formula OsO4. The compound is noteworthy for its many uses, despite its toxicity and the rarity of osmium. It also has a number of unusual properties, one being that the solid is volatile. The compound is colourless, but most samples appear yellow. This is most likely due to the presence of the impurity OsO2, which is yellow-brown in colour. In biology, its property of binding to lipids has made it a widely-used stain in electron microscopy. Physical properties Osmium(VIII) oxide forms monoclinic crystals. It has a characteristic acrid chlorine-like odor. The element name osmium is derived from ''osme'', Greek for ''odor''. OsO4 is volatile: it sublimes at room temperature. It is soluble in a wide range of organic solvents. It is also moderately soluble in water, with which it reacts reversibly to form osmic acid (see below). ''Pure'' osmium(VIII) oxide is probably colourless and it has been sugge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Permanganate
A permanganate () is a chemical compound containing the manganate(VII) ion, , the conjugate base of permanganic acid. Because the manganese atom is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion is a transition metal oxo complex with tetrahedral geometry. Permanganate solutions are purple in color and are stable in neutral or slightly alkaline media. The exact chemical reaction is dependent upon the organic contaminants present and the oxidant utilized. For example, trichloroethane (C2H3Cl3) is oxidized by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−). :8 + 3 → 6 + 8 + + 4 + 9 In an acidic solution, permanganate(VII) is reduced to the pale pink +2 oxidation state of the manganese(II) (Mn2+) ion. :8 + + 5 e− → Mn2+ + 4 H2O In a strongly basic solution, permanganate(VII) is reduced to the green +6 oxidation state of the manganate ion, . ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |