|
Martempering
Martempering is also known as stepped quenching or interrupted quenching. In this process, steel is heated above the upper critical point (above the transformation range) and then quenched in a hot-oil, molten-salt, or molten-lead bath kept at a temperature of 150-300 °C. The workpiece is maintained at a temperature above the martensite start (Ms) point until uniform temperature is achieved throughout its cross-section. It is then cooled to room temperature, typically in air or oil. The steel is then tempered. In this process, austenite is transformed to martensite by step quenching, at a rate fast enough to avoid the formation of ferrite, pearlite, or bainite. In the martempering process, austenitized metal part is immersed in a bath at a temperature just above the martensite start temperature (Ms). Interrupted quenching involves halting the cooling process at a temperature above the martensite transformation region, allowing sufficient time for the center of the workpiec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stepped Quenching
Tempering is a process of heat treating, which is used to increase the toughness of iron-based alloys. Tempering is usually performed after hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical point for a certain period of time, then allowing it to cool in still air. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, while springs are tempered at much higher temperatures. Introduction Tempering is a heat treatment technique applied to ferrous alloys, such as steel or cast iron, to achieve greater toughness by decreasing the hardness of the alloy. The reduction in hardness is usually accompanied by an increase in ductility, thereby decreasing the brittleness of the metal. Tempering is usually performed after quenchi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tempering (metallurgy)
Tempering is a process of heat treating, which is used to increase the toughness of iron-based alloys. Tempering is usually performed after Hardening (metallurgy), hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical point (thermodynamics), critical point for a certain period of time, then allowing it to cool in still air. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, while spring (device), springs are tempered at much higher temperatures. Introduction Tempering is a heat treatment technique applied to ferrous alloys, such as steel or cast iron, to achieve greater toughness by decreasing the hardness of the alloy. The reduction in hardness is usually accompanied by an increase in ductility, thereby decreasing the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic State of matter, states of matter: solid, liquid, and gas, and in rare cases, plasma (physics), plasma. A phase of a thermodynamic system and the states of matter have uniform physical property, physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molten Salt
Molten salt is salt which is solid at standard temperature and pressure but liquified due to elevated temperature. A salt that is liquid even at standard temperature and pressure is usually called a room-temperature ionic liquid, and molten salts are technically a class of ionic liquids. Examples As a reference, molten sodium chloride, table salt has a melting point (m.p.) of . A variety of eutectic mixtures have been developed with lower melting points: Chlorides *Lithium chloride and potassium chloride, m.p. . Nitrates Alkali metal nitrates are relatively low melting and thermally stable. The least stable, (m.p. ) decomposes only at . At the other extreme, cesium nitrate melts at and decomposes at 584 °C. *60:40 mixture of sodium nitrate and potassium nitrate is a liquid between . It has a heat of fusion of 161 J/g, and a heat capacity of 1.53 J/(g·K). *1:1 mixture :, m.p. . *40:7:53 ::, m. p. , stable to . Uses Molten salts have a variety of uses. Production of magnes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable nuclide, stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its Amphoterism, amphoteric nature; lead and lead oxides react with acids and base (chemistry), bases, and it tends to form covalent bonds. Lead compounds, Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Austenite
Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902). It exists at room temperature in some stainless steels due to the presence of nickel stabilizing the austenite at lower temperatures. Allotrope of iron From alpha iron undergoes a phase transition from body-centered cubic (BCC) to the face-centered cubic (FCC) configuration of gamma iron, also called austenite. This is similarly soft and ductile but can dissolve considerably more carbon (as much as 2.03% by mass at ). This gamma form of iron is present in the most commonly used type of stainless steel for making hospital and food-service equipment. Material Austenitiz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Martensite
Martensite is a very hard form of steel crystalline structure. It is named after German metallurgist Adolf Martens. By analogy the term can also refer to any crystal structure that is formed by diffusionless transformation. Properties Martensite is formed in carbon steels by the rapid cooling ( quenching) of the austenite form of iron at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite (Fe3C). Austenite is gamma-phase iron (γ-Fe), a solid solution of iron and alloying elements. As a result of the quenching, the face-centered cubic austenite transforms to a highly strained body-centered tetragonal form called martensite that is supersaturated with carbon. The shear deformations that result produce a large number of dislocations, which is a primary strengthening mechanism of steels. The highest hardness of a pearlitic steel is 400 Brinell, whereas martensite can achieve 700&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferrite (iron)
At atmospheric pressure, three allotropic forms of iron exist, depending on temperature: alpha iron (α-Fe, ferrite), gamma iron (γ-Fe, austenite), and delta iron (δ-Fe, similar to alpha iron). At very high pressure, a fourth form exists, epsilon iron (ε-Fe, hexaferrum). Some controversial experimental evidence suggests the existence of a fifth high-pressure form that is stable at very high pressures and temperatures. The phases of iron at atmospheric pressure are important because of the differences in solubility of carbon, forming different types of steel. The high-pressure phases of iron are important as models for the solid parts of planetary cores. The inner core of the Earth is generally assumed to consist essentially of a crystalline iron-nickel alloy with ε structure. The outer core surrounding the solid inner core is believed to be composed of liquid iron mixed with nickel and trace amounts of lighter elements. Standard pressure allotropes Alpha iron (α-Fe) Be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearlite
Pearlite is a two-phased, lamellar (or layered) structure composed of alternating layers of ferrite (87.5 wt%) and cementite (12.5 wt%) that occurs in some steels and cast irons. During slow cooling of an iron-carbon alloy, pearlite forms by a eutectoid reaction as austenite cools below (the eutectoid temperature). Pearlite is a microstructure occurring in many common grades of steels. Composition The eutectoid composition of austenite is approximately 0.8% carbon; steel with less carbon content ( hypoeutectoid steel) will contain a corresponding proportion of relatively pure ferrite crystallites that do not participate in the eutectoid reaction and cannot transform into pearlite. Likewise steels with higher carbon content ( hypereutectoid steels) will form cementite before reaching the eutectoid point. The proportion of ferrite and cementite forming above the eutectoid point can be calculated from the iron/iron—carbide equilibrium phase diagram using the lever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
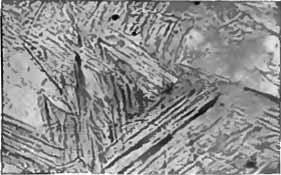
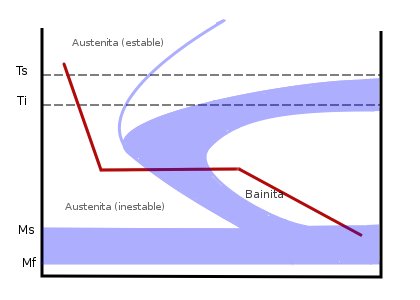
Bainite
Bainite is a plate-like microstructure that forms in steels at temperatures of 125–550 °C (depending on alloy content). First described by E. S. Davenport and Edgar Bain, it is one of the products that may form when austenite (the face-centered cubic crystal structure of iron) is cooled past a temperature where it is no longer thermodynamically stable with respect to ferrite, cementite, or ferrite and cementite. Davenport and Bain originally described the microstructure as similar in appearance to tempered martensite. A fine non- lamellar structure, bainite commonly consists of cementite and dislocation-rich ferrite. The large density of dislocations in the ferrite present in bainite, and the fine size of the bainite platelets, makes this ferrite harder than it normally would be. The temperature range for transformation of austenite to bainite (125–550 °C) is between those for pearlite and martensite. In fact, there is no fundamental lower limit to the bain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Austempering
Austempering is heat treatment that is applied to ferrous metals, most notably steel and ductile iron. In steel it produces a bainite microstructure whereas in cast irons it produces a structure of acicular ferrite and high carbon, stabilized austenite known as ''ausferrite''. It is primarily used to improve mechanical properties or reduce / eliminate distortion. Austempering is defined by both the process and the resultant microstructure. Typical austempering process parameters applied to an unsuitable material will not result in the formation of bainite or ausferrite and thus the final product will not be called austempered. Both microstructures may also be produced via other methods. For example, they may be produced as-cast or air cooled with the proper alloy content. These materials are also not referred to as austempered. History The austempering of steel was first pioneered in the 1930s by Edgar C. Bain and Edmund S. Davenport, who were working for the United States Stee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |