|
Kynapcin
Skeletal formula of kynapcin-24 Kynapcin is a general name for a number of dibenzofuranyl derivatives of the molecule polyozellin, present in the fungus ''Polyozellus multiplex''. Like polyozellin, some kynapcins inhibit prolyl endopeptidase, an enzyme that has a role in processing proteins including amyloid precursor protein Amyloid-beta precursor protein (APP) is an integral membrane protein expressed in many biological tissue, tissues and concentrated in the synapses of neurons. It functions as a cell surface receptor and has been implicated as a regulator .... Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects. Several kynapcins have been found in ''P. multiplex'', each with different chemical properties, including kynapcin-12, kynapcin-13 and -28, and -24. A total synthesis of kynapcin-24 was achieved in 2009. References Benzofurans Methyl esters Catechols {{organic-compound-s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyozellus Multiplex
''Polyozellus multiplex'' is a species of fungus first described in 1899, and is Common name, commonly known as the blue chanterelle, the clustered blue chanterelle, or, in Alaska, the black chanterelle. The distinctive Basidiocarp, fruit body of this species comprises blue- to purple-colored clusters of vase- or spoon-shaped Pileus (mycology), caps with veiny wrinkles on the undersurface that run down the length of the Stipe (mycology), stem. It was considered the monotypic species of ''Polyozellus'' until recent molecular work split the species complex into five species. The genus name is derived from the Greek language, Greek ''poly'' meaning ''many'', and ''oz'', meaning ''branch''. The Specific name (botany), specific epithet ''multiplex'' means "in many pieces", referring to the compound nature of the fruit body. ''P. multiplex'' may be found growing on the ground in Temperate coniferous forest, coniferous forests, usually under spruce and fir trees. It is an Edible mush ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibenzofuran
Dibenzofuran is a heterocyclic organic compound with the chemical structure shown at right. It is an aromatic compound that has two benzene rings fused to a central furan ring. All the numbered carbon atoms have a hydrogen atom bonded to each of them. It is a volatile white solid that is soluble in nonpolar organic solvents. It is obtained from coal tar, where it exists as a 1% component.Gerd Collin and Hartmut Höke "Benzofurans" in Ullmann's Encyclopedia of Industrial Chemistry, 2007, Wiley-VCH, Weinheim. Reactions Dibenzofuran is thermally robust with a convenient liquid range. These properties, together with its low toxicity, are exploited by the use of DBF as a heat transfer agent. It undergoes electrophilic reactions, such as halogenation and Friedel-Crafts reactions. Reaction of DBF with butyl lithium results in dilithiation.Ulrich Iserloh, Yoji Oderaotoshi, Shuji Kanemasa, and Dennis Patrick Curran, Dennis P. Curran "Synthesis of (R,R)-4,6-Dibenzofurandiyl-2,2'-Bis (4-P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is avai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyozellin
Polyozellin is a chemical which occurs in the mushroom '' Polyozellus multiplex''. It inhibits prolyl endopeptidase, an enzyme that has a role in processing proteins (specifically, amyloid precursor protein) in Alzheimer's disease. Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects. Structurally related dibenzofuranyl derivatives of polyozellin are known as kynapcin Skeletal formula of kynapcin-24 Kynapcin is a general name for a number of dibenzofuranyl derivatives of the molecule polyozellin, present in the fungus ''Polyozellus multiplex''. Like polyozellin, some kynapcins inhibit prolyl endopeptidase, a ...s. References Dibenzofurans Benzofuran ethers at the benzene ring Acetate esters Phenol esters Hydroxyquinol ethers {{Aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Inhibition
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which substrate molecules are converted into products. An enzyme facilitates a specific chemical reaction by binding the substrate to its active site, a specialized area on the enzyme that accelerates the most difficult step of the reaction. An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis of the reaction is blocked. Enzyme inhibitors may bind reversibly or irreversibly. Irreversible inhibitors form a chemical bond with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By contrast, reversible inhibitors bind non-covalently and may spontaneously leave the enzyme, allowing the enzyme to resume its function. Rev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prolyl Endopeptidase
Prolyl endopeptidase (PE) also known as prolyl oligopeptidase or post-proline cleaving enzyme is an enzyme that in humans is encoded by the ''PREP'' gene. Function Prolyl endopeptidase is a large cytosolic enzyme that belongs to a distinct class of serine peptidases. It was first described in the cytosol of rabbit brain as an oligopeptidase, which degrades the nonapeptide bradykinin at the Pro-Phe bond. The enzyme is involved in the maturation and degradation of peptide hormones and neuropeptides such as alpha-melanocyte-stimulating hormone, luteinizing hormone-releasing hormone (LH-RH), thyrotropin-releasing hormone, angiotensin, neurotensin, oxytocin, substance P and vasopressin. PREP cleaves peptide bonds at the C-terminal side of proline residues. Its activity is confined to action on oligopeptides of less than 10 kD and it has an absolute requirement for the trans-configuration of the peptide bond preceding proline. Prolyl endopeptidases are involved in the maturation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are Ribozyme, catalytic RNA molecules, called ribozymes. Enzymes' Chemical specificity, specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyloid Precursor Protein
Amyloid-beta precursor protein (APP) is an integral membrane protein expressed in many biological tissue, tissues and concentrated in the synapses of neurons. It functions as a cell surface receptor and has been implicated as a regulator of synapse formation, neural plasticity, antimicrobial activity, and iron export. It is coded for by the gene ''APP'' and regulated by substrate presentation. APP is best known as the precursor molecule whose proteolysis generates amyloid beta (Aβ), a polypeptide containing 37 to 49 amino acid residues, whose Amyloid#Structure, amyloid fibrillar form is the primary component of amyloid plaques found in the brains of Alzheimer's disease patients. Genetics Amyloid-beta precursor protein is an ancient and highly Conserved sequence, conserved protein. In humans, the gene ''APP'' is located on chromosome 21 and contains 18 exons spanning 290 kilobases. Several alternative splicing isoforms of APP have been observed in humans, ranging in len ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Property
A chemical property is any of a material's properties that becomes evident during, or after, a chemical reaction; that is, any quality that can be established only by changing a substance's chemical identity.William L. Masterton, Cecile N. Hurley, "Chemistry: Principles and Reactions", 6th edition. Brooks/Cole Cengage Learning, 2009, p.1(Google books)/ref> Simply speaking, chemical properties cannot be determined just by viewing or touching the substance; the substance's internal structure must be affected greatly for its chemical properties to be investigated. When a substance goes under a chemical reaction, the properties will change drastically, resulting in chemical change. However, a catalytic property would also be a chemical property. Chemical properties can be contrasted with physical properties, which can be discerned without changing the substance's structure. However, for many properties within the scope of physical chemistry, and other disciplines at the boundary b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes it from semisynthesis. Syntheses may sometimes conclude at a precursor with further known synthetic pathways to a target molecule, in which case it is known as a formal synthesis. Total synthesis target molecules can be natural products, medicinally-important active ingredients, known intermediates, or molecules of theoretical interest. Total synthesis targets can also be organometallic or inorganic, though these are rarely encountered. Total synthesis projects often require a wide diversity of reactions and reagents, and subsequently requires broad chemical knowledge and training to be successful. Often, the aim is to discover a new route of synthesis for a target molecule for which there already exist known routes. Sometimes, however, no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
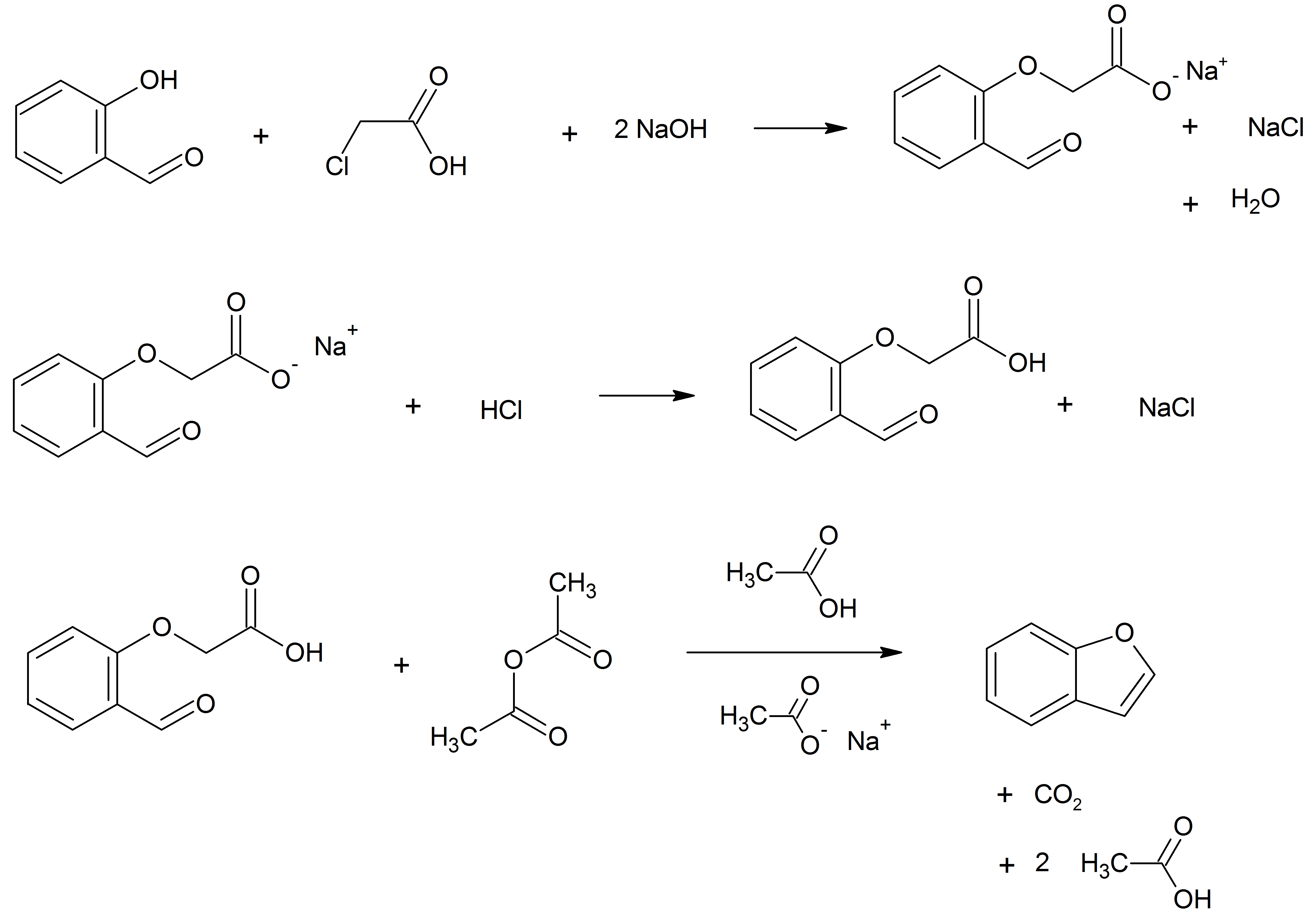
Benzofurans
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px *Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |