|
Glycidol
Glycidol is an organic compound with the formula . The molecule contains both epoxide and alcohol functional groups. Being simple to make and bifunctional, it has a variety of industrial uses. The compound is a colorless, slightly viscous liquid that is slightly unstable and is not often encountered in pure form. Synthesis and applications Glycidol is prepared by the epoxidation of allyl alcohol. A typical catalyst is tungstic acid, and a typical O-atom source is aqueous peroxyacetic acid.Guenter Sienel, Robert Rieth, Kenneth T. Rowbottom "Epoxides" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Some useful products derived from glycidol are 2,3-epoxypropyloxy chloroformate (from phosgene) and glycidyl urethanes (by addition of isocyanates): : : Glycidol is used as a chemical intermediate in the synthesis of other glycidyl ethers, esters, and amines. Glycidol can be O-benzylated in the presence of strong base. More typically, such glycidol e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxide
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile. Nomenclature A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound ''1,2-epoxyheptane'', which can also be called ''1,2-heptene oxide''. A polymer formed from epoxide precursors is called an ''epoxy''. However, few if any of the epoxy groups i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxides
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom Ring (chemistry), ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly Reactivity (chemistry), reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often Volatility (chemistry), volatile. Nomenclature A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound ''1,2-epoxyheptane'', which can also be called ''1,2-heptene oxide''. A polymer formed from epoxide precursors is c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of IARC Group 2A Agents - Probably Carcinogenic To Humans
IARC group 2A agents are substances and exposure circumstances that have been classified as probable carcinogens by the International Agency for Research on Cancer (IARC). This designation is applied when there is limited evidence of carcinogenicity in humans, as well as ''sufficient evidence'' of carcinogenicity in experimental animals. In some cases, an agent may be classified in this group when there is ''inadequate evidence'' of carcinogenicity in humans along with ''sufficient evidence'' of carcinogenicity in experimental animals and ''strong evidence'' that the carcinogenesis is mediated by a mechanism that also operates in humans. Exceptionally, an agent may be classified in this group solely on the basis of ''limited evidence'' of carcinogenicity in humans. This list is focusing on the hazard linked to the agents. This means that the carcinogenic agents are capable of causing cancer, but this does not take their risk into account, which is the probability of causing a can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Cosmetics Toxicology
''Food and Chemical Toxicology'' is a peer-reviewed scientific journal covering aspects of food safety, chemical safety, and other aspects of consumer product safety. It is published by Elsevier and was established in 1963. The editor-in-chief is Bryan Delaney. Abstracting and indexing The journal is abstracted and indexed in Analytical Abstracts, BIOSIS Previews, CAB International, Chemical Abstracts Service, Current Contents/Agriculture, Biology & Environmental Sciences, Current Contents/Life Sciences, Elsevier BIOBASE, EMBASE, MEDLINE/PubMed, Science Citation Index, and Scopus. According to the ''Journal Citation Reports'', it has a 2014 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a type of journal ranking. Journals with higher impact factor values are considered more prestigious or important within their field. The Impact Factor of a journa ... of 2.895, ranking it 30th out of 87 journals in the category "Toxicology" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
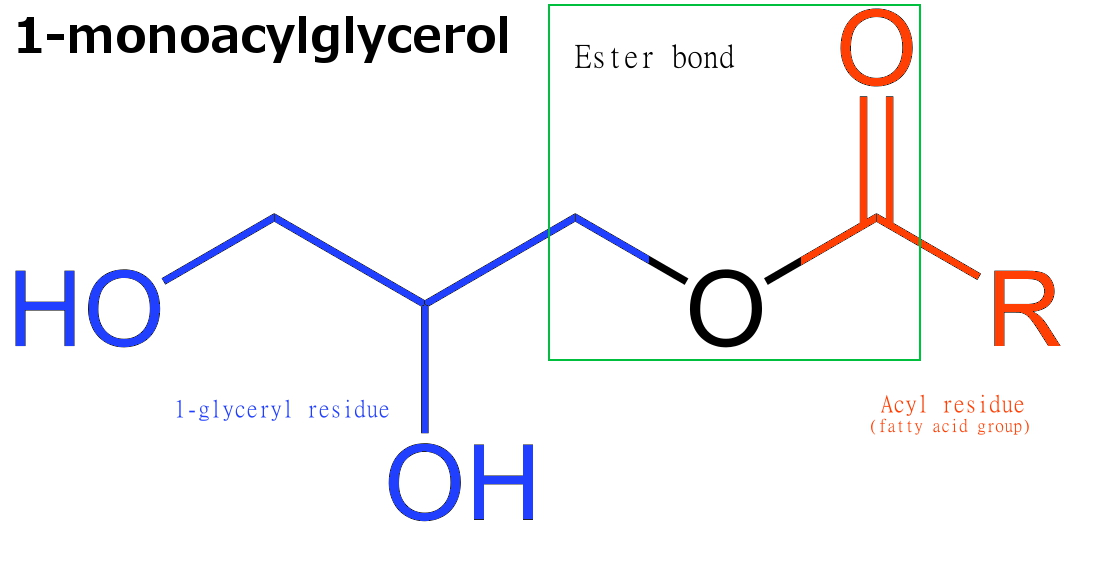
Monoglyceride
Monoglycerides (also: acylglycerols or monoacylglycerols) are a class of glycerides which are composed of a molecule of glycerol linked to a fatty acid via an ester bond. As glycerol contains both primary and secondary alcohol groups two different types of monoglycerides may be formed; 1-monoacylglycerols where the fatty acid is attached to a primary alcohol, or a 2-monoacylglycerols where the fatty acid is attached to the secondary alcohol. Synthesis Monoglycerides are produced both biologically and industrially. They are naturally present at very low levels (0.1-0.2%) in some seed oils such as olive oil, rapeseed oil and cottonseed oil. They are biosynthesized by the enzymatic hydrolysis of triglycerides by lipoprotein lipase and the enzymatic hydrolysis of diglycerides by diacylglycerol lipase; or as an intermediate in the alkanoylation of glycerol to form fats. Several monoglycerides are pharmacologically active (e.g. 2-oleoylglycerol, 2-arachidonoylglycerol). Industrial pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycidic Acid
Glycidic acid is an organic compound that has both epoxide and carboxylic acid functions. It may be prepared by the oxidation of glycidol, or by the epoxidation of acrylic acid Acrylic acid (IUPAC: prop-2-enoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has .... This compound is commercially available as well. See also * Glycidamide References {{reflist Carboxylic acids Epoxides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycidamide
Glycidamide is an organic compound with the formula H2NC(O)C2H3O. It is a colorless oil. Structurally, it contains adjacent amides and epoxide functional groups. It is a bioactive, potentially toxic or even carcinogenic metabolite of acrylonitrile and acrylamide. It is a chiral molecule. Structure and reactivity Glycidamide is a reactive epoxide metabolite from acrylamideBergmark, E., Calleman, C. J., & Costa, L. G. (1991). Formation of hemoglobin adducts of acrylamide and its epoxide metabolite glycidamide in the rat. Toxicology and Applied Pharmacology, 111(2), 352-363.Beland, F. A., Olson, G. R., Mendoza, M. C., Marques, M. M., & Doerge, D. R. (2015). Carcinogenicity of glycidamide in B6C3F 1 mice and F344/N rats from a two-year drinking water exposure. Food and Chemical Toxicology, 86, 104-115. and can react with nucleophiles. This results in covalent binding of the electrophile. Glycidamide gives a positive response in the Ames/Salmonella mutagenicity assay, which indic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Oxide
Ethylene oxide is an organic compound with the chemical formula, formula . It is a cyclic ether and the simplest epoxide: a three-membered ring (chemistry), ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of a silver catalyst. The reactivity that is responsible for many of ethylene oxide's hazards also makes it useful. Although too dangerous for direct household use and generally unfamiliar to consumers, ethylene oxide is used for making many consumer products as well as non-consumer chemicals and intermediates. These products include detergents, thickeners, solvents, plastics, and various organic chemicals such as ethylen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epichlorohydrin
Epichlorohydrin (abbreviated ECH) is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscibility, miscible with most polar molecule, polar Organic compound, organic solvents. It is a chiral molecule generally existing as a racemic mixture of right-handed and left-handed enantiomers. Epichlorohydrin is a highly reactive electrophile, electrophilic compound and is used in the production of glycerol, plastics, Epoxy resin, epoxy glues and resins, epoxy diluents and elastomers. Production Epichlorohydrin is traditionally manufactured from allyl chloride in two steps, beginning with the addition of hypochlorous acid, which affords a mixture of two structural isomer, isomeric alcohols: : In the second step, this mixture is treated with base to give the epoxide: : In this way, more than 800,000 tons (1997) of epichlorohydrin are produced annually. Glycer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recommended Exposure Limit
A recommended exposure limit (REL) is an occupational exposure limit that has been recommended by the United States National Institute for Occupational Safety and Health. The REL is a level that NIOSH believes would be protective of worker safety and health over a working lifetime if used in combination with engineering and work practice controls, exposure and medical monitoring, posting and labeling of hazards, worker training and personal protective equipment. To formulate these recommendations, NIOSH evaluates all known and available medical, biological, engineering, chemical, trade, and other information. Although not legally enforceable limits, RELS are transmitted to the Occupational Safety and Health Administration (OSHA) or the Mine Safety and Health Administration (MSHA) of the U.S. Department of Labor for use in promulgating legal standards. All RELs are located in the ''NIOSH Pocket Guide to Chemical Hazards'', along with other key data for 677 chemical or substance gro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Institute For Occupational Safety And Health
The National Institute for Occupational Safety and Health (NIOSH, ) is the List of United States federal agencies, United States federal agency responsible for conducting research and making recommendations for the prevention of work-related occupational injury, injury, occupational disease, illness, disability, and occupational fatality, death. Its functions include gathering information, conducting scientific research both in the laboratory and in the field, and translating the knowledge gained into products and services.About NIOSH National Institute for Occupational Safety and Health. Among NIOSH's programs are determination of recommended exposure limits for toxic chemicals and other hazards, field research such as the Health Hazard Evaluation Program, epidemiology and health surveillance programs such as the National Firefighter Re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Permissible Exposure Limit
The permissible exposure limit (PEL or OSHA PEL) is a legal limit in the United States for exposure of an employee to a chemical substance or physical agents such as high level noise. Permissible exposure limits were established by the Occupational Safety and Health Administration (OSHA). Most of OSHA's PELs were issued shortly after the adoption of the Occupational Safety and Health (OSH) Act in 1970. Chemical regulation is sometimes expressed in parts per million (ppm), but often in milligrams per cubic meter (mg/m3). Units of measure for physical agents such as noise are specific to the agent. A PEL is usually given as a time-weighted average (TWA), although some are short-term exposure limits (STEL) or ceiling limits. A TWA is the average exposure over a specified period, usually a nominal eight hours. This means that for limited periods, a worker may be exposed to concentration excursions higher than the PEL as long as the TWA is not exceeded and any applicable excursion l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |