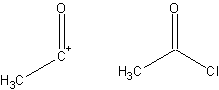|
Ethylmagnesium Bromide
Ethylmagnesium bromide is a Grignard reagent with formula , often abbreviated to , where Et is ethyl group. It is widely used in the laboratory synthesis of organic compounds. Reactions Apart from acting as the synthetic equivalent of an ethyl anion synthon for nucleophilic addition, ethylmagnesium bromide may be used as a strong base to deprotonate various substrates such as alkynes: :RC≡CH + EtMgBr → RC≡CMgBr + EtH In this application, ethylmagnesium bromide has been supplanted by the wide availability of organolithium reagents. Preparation Ethylmagnesium bromide is commercially available, usually as a solution in diethyl ether or tetrahydrofuran. It may be prepared in the normal manner of Grignard reagents — by reacting bromoethane with magnesium in diethyl ether Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("et ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon–carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. Grignard reagents are rarely isolated as solids. Instead, they are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran using air-free techniques. Grignard reagents are complex with the magnesium atom bonded to two ether ligands as well as the halide and organyl ligands. The discovery of the Grignard reaction in 1900 was recogn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Group
In organic chemistry, an ethyl group (abbr. Et) is an alkyl substituent with the formula , derived from ethane (). ''Ethyl'' is used in the International Union of Pure and Applied Chemistry The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...'s nomenclature of organic chemistry for a saturated two-carbon moiety in a molecule, while the prefix "''eth-''" is used to indicate the presence of two carbon atoms in the molecule. Ethylation Ethylation is the formation of a compound by introduction of the ethyl group. The most widely practiced example of this reaction is the ethylation of benzene with ethylene to yield ethylbenzene, a precursor to styrene, which is a precursor to polystyrene. Approximately 24.7 million tons of ethylbenzene were produced in 1999. :: Many ethyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Equivalent
Retrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures regardless of any potential reactivity/interaction with reagents. Each precursor material is examined using the same method. This procedure is repeated until simple or commercially available structures are reached. These simpler/commercially available compounds can be used to form a synthesis of the target molecule. Retrosynthetic analysis was used as early as 1917 in Robinson's Tropinone total synthesis. Important conceptual work on retrosynthetic analysis was published by George Vladutz in 1963. E.J. Corey formalized and popularized the concept from 1967 onwards in his article ''General methods for the construction of complex molecules'' and his book ''The Logic of Chemical Synthesis''. The power of retrosynthetic analysis becomes evident in the design of a synthesis. The goal of retrosynthetic ana ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthon
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 1988 that the "word ''synthon'' has now come to be used to mean synthetic ''building block'' rather than retrosynthetic fragmentation structures". It was noted in 1998 that the phrase did not feature very prominently in Corey's 1981 book ''The Logic of Chemical Synthesis'', as it was not included in the index. Because synthons are Ion, charged, when placed into a synthesis an uncharged form is found commercially instead of forming and using the potentially very unstable charged synthons. Example : In planning the synthesis of phenylacetic acid, two synthons are identified: a nucleophilic "COOH−" group, and an electrophilic "PhCH2+" group. Of course, both synthons do not exist by themselves; synthetic equivalents corresponding to the synthons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions differ from electrophilic additions in that the former reactions involve the group to which atoms are added accepting electron pairs, whereas the latter reactions involve the group donating electron pairs. Addition to carbon–heteroatom double bonds Nucleophilic addition reactions of nucleophiles with electrophilic double or triple bond (π bonds) create a new carbon center with two additional single, or σ, bonds.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Addition of a nucleophile to carbon–heteroatom double or triple bonds such as >C=O or -C≡N show great variety. These types of bonds are polar (have a large difference in electronegativit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethane
Ethane ( , ) is a naturally occurring Organic compound, organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is List of purification methods in chemistry, isolated on an industrial scale from natural gas and as a petrochemical by-product of oil refinery, petroleum refining. Its chief use is as feedstock for ethylene production. The ethyl group is formally, although rarely practically, derived from ethane. History Ethane was first synthesised in 1834 by Michael Faraday, applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane and did not investigate it further. The process is now called Kolbe electrolysis: : acetate, CH3COO− → CH3• + carbon dioxide, CO2 + electron, e− : CH3• + •CH3 → C2H6 During the period 1847–1849, in an effort to vindicate the radical theory of organic chemistry, Hermann Kolbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organolithium Reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. History and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs to the ether class of organic compounds. It is a common solvent and was formerly used as a general anesthetic. Production Most diethyl ether is produced as a byproduct of the vapor-phase Hydration reaction, hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid Catalysis, catalysts and can be adjusted to make more ether if the need arises: Vapor-phase Dehydration reaction, dehydration of ethanol over some Aluminium oxide, alumina catalysts can give diethyl ether yields of up to 95%. : Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Uses The dominant use of diethyl ether is as a solvent. One particular application is in the production of cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. It is an isomer of another solvent, butanone. Production About 200,000 tonnes of tetrahydrofuran are produced annually. The most widely used industrial process involves the acid-catalyzed dehydration of 1,4-Butanediol, 1,4-butanediol. Ashland Inc., Ashland/ISP is one of the biggest producers of this chemical route. The method is similar to the production of diethyl ether from ethanol. The butanediol is derived from Condensation reaction, condensation of acetylene with formaldehyde followed by hydrogenation. DuPont developed a process for producing THF by oxidizing Butane#Isomers, ''n''-butane to crude maleic anhydride, follow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoethane
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr (which is also used as an abbreviation for ethidium bromide). This volatile compound has an ether-like odor. Preparation The preparation of EtBr stands as a model for the synthesis of bromoalkanes in general. It is usually prepared by the addition of hydrogen bromide to ethene: :H2C=CH2 + HBr → H3C-CH2Br Bromoethane is inexpensive and would rarely be prepared in the laboratory. A laboratory synthesis includes reacting ethanol with a mixture of hydrobromic and sulfuric acids. An alternate route involves refluxing ethanol with phosphorus and bromine; phosphorus tribromide is generated ''in situ''. Uses In organic synthesis, EtBr is the synthetic equivalent of the ethyl carbocation (Et+) synthon. In reality, such a cation is not actually formed. For example, carboxylates salts are converted to ethyl esters, carbanions to ethylated derivatives, thioure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |