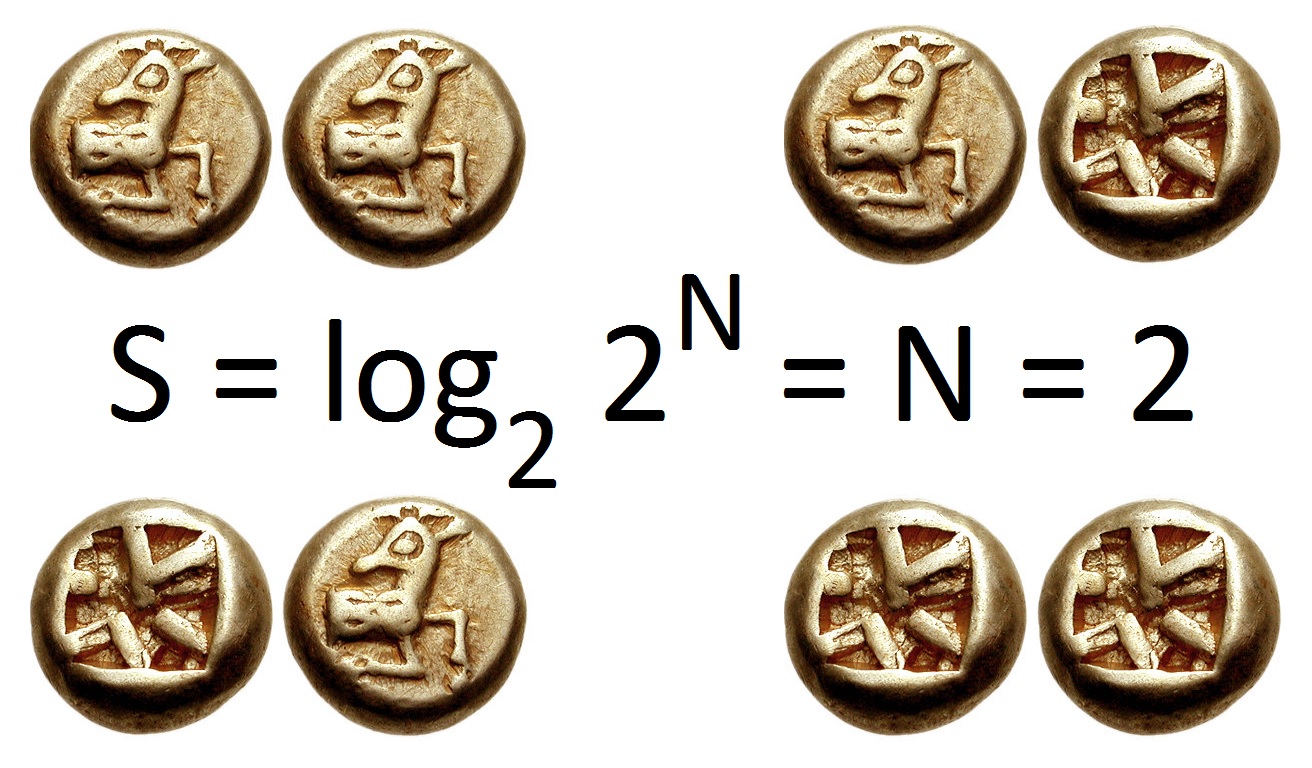|
Entropy Of Mixing
In thermodynamics, the entropy of mixing is the increase in the total entropy when several initially separate systems of different composition, each in a thermodynamic state of internal equilibrium, are mixed without chemical reaction by the thermodynamic operation of removal of impermeable partition(s) between them, followed by a time for establishment of a new thermodynamic state of internal equilibrium in the new unpartitioned closed system. In general, the mixing may be constrained to occur under various prescribed conditions. In the customarily prescribed conditions, the materials are each initially at a common temperature and pressure, and the new system may change its volume, while being maintained at that same constant temperature, pressure, and chemical component masses. The volume available for each material to explore is increased, from that of its initially separate compartment, to the total common final volume. The final volume need not be the sum of the initially se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantity, physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the thermodynamic efficiency, efficiency of early steam engines, particularly through the work of French physicist Nicolas Léonard Sadi Carnot, Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regular Solution
In chemistry, a regular solution is a solution whose entropy of mixing is equal to that of an ideal solution with the same composition, but is non-ideal due to a nonzero enthalpy of mixing.P. Atkins and J. de Paula, ''Atkins' Physical Chemistry'' (8th ed. W.H. Freeman 2006) p.149P.A. Rock, ''Chemical Thermodynamics. Principles and Applications'' (Macmillan 1969) p.263 Such a solution is formed by random mixing of components of similar molar volume and without strong specific interactions, and its behavior diverges from that of an ideal solution by showing phase separation at intermediate compositions and temperatures (a miscibility gap). Its entropy of mixing is equal to that of an ideal solution with the same composition, due to random mixing without strong specific interactions. For two components :\Delta S_ = -nR(x_1\ln x_1 + x_2\ln x_2)\, where R\, is the gas constant, n\, the total number of mole (unit), moles, and x_i\, the mole fraction of each component. Only the enthalpy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy (information Theory)
In information theory, the entropy of a random variable quantifies the average level of uncertainty or information associated with the variable's potential states or possible outcomes. This measures the expected amount of information needed to describe the state of the variable, considering the distribution of probabilities across all potential states. Given a discrete random variable X, which may be any member x within the set \mathcal and is distributed according to p\colon \mathcal\to[0, 1], the entropy is \Eta(X) := -\sum_ p(x) \log p(x), where \Sigma denotes the sum over the variable's possible values. The choice of base for \log, the logarithm, varies for different applications. Base 2 gives the unit of bits (or "shannon (unit), shannons"), while base Euler's number, ''e'' gives "natural units" nat (unit), nat, and base 10 gives units of "dits", "bans", or "Hartley (unit), hartleys". An equivalent definition of entropy is the expected value of the self-information of a v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy (statistical Thermodynamics)
The concept entropy was first developed by German physicist Rudolf Clausius in the mid-nineteenth century as a thermodynamic property that predicts that certain spontaneous processes are irreversible or impossible. In statistical mechanics, entropy is formulated as a statistical property using probability theory. The statistical entropy perspective was introduced in 1870 by Austrian physicist Ludwig Boltzmann, who established a new field of physics that provided the descriptive linkage between the macroscopic observation of nature and the microscopic view based on the rigorous treatment of large ensembles of microscopic states that constitute thermodynamic systems. Boltzmann's principle Ludwig Boltzmann defined entropy as a measure of the number of possible microscopic states (''microstates'') of a system in thermodynamic equilibrium, consistent with its macroscopic thermodynamic properties, which constitute the ''macrostate'' of the system. A useful illustration is the example ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Critical Point (thermodynamics)
In thermodynamics, a critical point (or critical state) is the end point of a phase Equilibrium (thermodynamics), equilibrium curve. One example is the liquid–vapor critical point, the end point of the pressure–temperature curve that designates conditions under which a liquid and its vapor can coexist. At higher temperatures, the gas comes into a supercritical fluid, supercritical phase, and so cannot be liquefied by pressure alone. At the critical point, defined by a ''critical temperature'' ''T''c and a ''critical pressure'' ''p''c, phase (matter), phase boundaries vanish. Other examples include the Upper critical solution temperature, liquid–liquid critical points in mixtures, and the ferromagnet–paramagnet transition (Curie temperature) in the absence of an external magnetic field. Liquid–vapor critical point Overview For simplicity and clarity, the generic notion of ''critical point'' is best introduced by discussing a specific example, the vapor–liquid cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexane is mainly used for the industrial production of adipic acid and caprolactam, which are Precursor (chemistry), precursors to nylon. Cyclohexyl () is the alkyl substituent of cyclohexane and is abbreviated Cy. Production Cyclohexane is one of components of naphtha, from which it can be extracted by advanced distillation methods. Distillation is usually combined with isomerization of methylcyclopentane, a similar component extracted from naphtha by similar methods. Together, these processes cover only a minority (15-20%) of the modern industrial demand, and are complemented by synthesis. Modern industrial synthesis On an industrial scale, cyclohexane is produced by hydrogenation of benzene in the presence of a Raney nickel catalyst. Prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a poor barrier to air and water vapor and has a relatively low melting point. Polystyrene is one of the most widely used plastics, with the scale of its production being several million tonnes per year. Polystyrene is naturally transparent to visible light, but can be colored with colorants. Uses include protective packaging (such as packing peanuts and optical disc jewel cases), containers, lids, bottles, trays, tumblers, disposable cutlery, in the making of models, and as an alternative material for phonograph records. As a thermoplastic polymer, polystyrene is in a solid (glassy) state at room temperature but flows if heated above about 100 °C, its glass transition temperature. It becomes rigid again when cooled. This te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyacrylic Acid
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2−CHCO2H)''n''. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deprotonated derivatives thereof, are known and of commercial value. In a water solution at neutral pH, PAA is an anionic polymer, i.e., many of the side chains of PAA lose their protons and acquire a negative charge. Partially or wholly deprotonated PAAs are polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. These properties acid–base and water-attracting are the basis of many applications. Synthesis PAA, like any acrylate polymer, is usually synthesized through a process known as free radical polymerization, though graft polymerization may also be used. Free radical polymerization involves the conversion of monomers, in this case, acrylic acid (CH2=CHCO2H), into a polymer cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently bonded to a more Electronegativity, electronegative donor atom or group (Dn), interacts with another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Unlike simple Dipole–dipole attraction, dipole–dipole interactions, hydrogen bonding arises from charge transfer (nB → σ*AH), Atomic orbital, orbital interactions, and quantum mechanical Delocalized electron, delocalization, making it a resonance-assisted interaction rather than a mere electrostatic attraction. The general notation for hydrogen bonding is Dn−H···Ac, where the solid line represents a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are nitrogen (N), oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base. Synthesis and properties Triethylamine is prepared by the alkylation of ammonia with ethanol: :NH3 + 3 C2H5OH → N(C2H5)3 + 3 H2O The pKa of protonated triethylamine is 10.75,David Evans Research Group and it can be used to prepare buffer solutions at that pH. The hydrochloride |