|
Entropy (statistical Thermodynamics)
The concept entropy was first developed by German physicist Rudolf Clausius in the mid-nineteenth century as a thermodynamic property that predicts that certain spontaneous processes are irreversible or impossible. In statistical mechanics, entropy is formulated as a statistical property using probability theory. The statistical entropy perspective was introduced in 1870 by Austrian physicist Ludwig Boltzmann, who established a new field of physics that provided the descriptive linkage between the macroscopic observation of nature and the microscopic view based on the rigorous treatment of large ensembles of microscopic states that constitute thermodynamic systems. Boltzmann's principle Ludwig Boltzmann defined entropy as a measure of the number of possible microscopic states (''microstates'') of a system in thermodynamic equilibrium, consistent with its macroscopic thermodynamic properties, which constitute the ''macrostate'' of the system. A useful illustration is the example ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rudolf Clausius
Rudolf Julius Emanuel Clausius (; 2 January 1822 – 24 August 1888) was a German physicist and mathematician and is considered one of the central founding fathers of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he gave the theory of heat a truer and sounder basis. His most important paper, "On the Moving Force of Heat", published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy. In 1870 he introduced the virial theorem, which applied to heat. Life Clausius was born in Köslin (now Koszalin, Poland) in the Province of Pomerania in Prussia. His father was a Protestant pastor and school inspector, and Rudolf studied in the school of his father. In 1838, he went to the Gymnasium in Stettin. Clausius graduated from the University of Berlin in 1844 where he had studied mathematics and physics since 1840 with, among others, Gustav Magnus, Peter Gusta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Logarithm
The natural logarithm of a number is its logarithm to the base of a logarithm, base of the e (mathematical constant), mathematical constant , which is an Irrational number, irrational and Transcendental number, transcendental number approximately equal to . The natural logarithm of is generally written as , , or sometimes, if the base is implicit, simply . Parentheses are sometimes added for clarity, giving , , or . This is done particularly when the argument to the logarithm is not a single symbol, so as to prevent ambiguity. The natural logarithm of is the exponentiation, power to which would have to be raised to equal . For example, is , because . The natural logarithm of itself, , is , because , while the natural logarithm of is , since . The natural logarithm can be defined for any positive real number as the Integral, area under the curve from to (with the area being negative when ). The simplicity of this definition, which is matched in many other formulas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
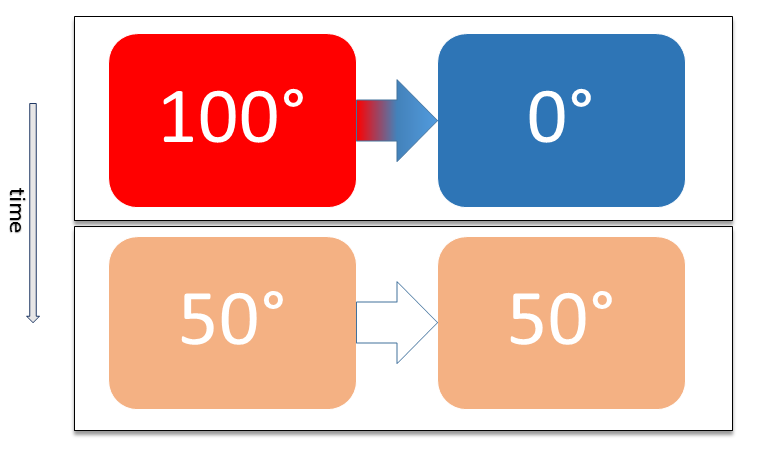
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on Universal (metaphysics), universal empirical observation concerning heat and Energy transformation, energy interconversions. A simple statement of the law is that heat always flows spontaneously from hotter to colder regions of matter (or 'downhill' in terms of the temperature gradient). Another statement is: "Not all heat can be converted into Work (thermodynamics), work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system. It predicts whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. For example, the first law allows the process of a cup falling off a table and breaking on the floor, as well as allowi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistical Ensemble
In physics, specifically statistical mechanics, an ensemble (also statistical ensemble) is an idealization consisting of a large number of virtual copies (sometimes infinitely many) of a system, considered all at once, each of which represents a possible state that the real system might be in. In other words, a statistical ensemble is a set of systems of particles used in statistical mechanics to describe a single system. The concept of an ensemble was introduced by J. Willard Gibbs in 1902. A thermodynamic ensemble is a specific variety of statistical ensemble that, among other properties, is in statistical equilibrium (defined below), and is used to derive the properties of thermodynamic systems from the laws of classical or quantum mechanics. Physical considerations The ensemble formalises the notion that an experimenter repeating an experiment again and again under the same macroscopic conditions, but unable to control the microscopic details, may expect to observe a ran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isolated System
In physical science, an isolated system is either of the following: # a physical system so far removed from other systems that it does not interact with them. # a thermodynamic system enclosed by rigid immovable walls through which neither mass nor energy can pass. Though subject internally to its own gravity, an isolated system is usually taken to be outside the reach of external gravitational and other long-range forces. This can be contrasted with what (in the more common terminology used in thermodynamics) is called a closed system, being enclosed by selective walls through which energy can pass as heat or work, but not matter; and with an open system, which both matter and energy can enter or exit, though it may have variously impermeable walls in parts of its boundaries. An isolated system obeys the conservation law that its total energy–mass stays constant. Most often, in thermodynamics, mass and energy are treated as separately conserved. Because of the requir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in thermal equilibrium with itself if the temperature within the system is spatially uniform and temporally constant. Systems in thermodynamic equilibrium are always in thermal equilibrium, but the converse is not always true. If the connection between the systems allows transfer of energy as 'change in internal energy' but does not allow transfer of matter or transfer of energy as work, the two systems may reach thermal equilibrium without reaching thermodynamic equilibrium. Two varieties of thermal equilibrium Relation of thermal equilibrium between two thermally connected bodies The relation of thermal equilibrium is an instance of equilibrium between two bodies, which means that it refers to transfer through a selectively permeable par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K). Heat capacity is an extensive property. The corresponding intensive property is the specific heat capacity, found by dividing the heat capacity of an object by its mass. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The volumetric heat capacity measures the heat capacity per volume. In architecture and civil engineering, the heat capacity of a building is often referred to as its '' thermal mass''. Definition Basic definition The heat capacity of an object, denoted by C, is the limit C = \lim_\frac, where \Delta Q is the amount of heat that must be added to the object (of mass ''M'') in order to raise its temperature by \Delta T. The value of this parameter usually varies considerably depending o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SI Unit
The International System of Units, internationally known by the abbreviation SI (from French ), is the modern form of the metric system and the world's most widely used system of units of measurement, system of measurement. It is the only system of measurement with official status in nearly every country in the world, employed in science, technology, industry, and everyday commerce. The SI system is coordinated by the International Bureau of Weights and Measures, which is abbreviated BIPM from . The SI comprises a coherence (units of measurement), coherent system of unit of measurement, units of measurement starting with seven SI base unit, base units, which are the second (symbol s, the unit of time), metre (m, length), kilogram (kg, mass), ampere (A, electric current), kelvin (K, thermodynamic temperature), mole (unit), mole (mol, amount of substance), and candela (cd, luminous intensity). The system can accommodate coherent units for an unlimited number of additional quantiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimensionless Quantity
Dimensionless quantities, or quantities of dimension one, are quantities implicitly defined in a manner that prevents their aggregation into unit of measurement, units of measurement. ISBN 978-92-822-2272-0. Typically expressed as ratios that align with another system, these quantities do not necessitate explicitly defined Unit of measurement, units. For instance, alcohol by volume (ABV) represents a volumetric ratio; its value remains independent of the specific Unit of volume, units of volume used, such as in milliliters per milliliter (mL/mL). The 1, number one is recognized as a dimensionless Base unit of measurement, base quantity. Radians serve as dimensionless units for Angle, angular measurements, derived from the universal ratio of 2π times the radius of a circle being equal to its circumference. Dimensionless quantities play a crucial role serving as parameters in differential equations in various technical disciplines. In calculus, concepts like the unitless ratios ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Summation
In mathematics, summation is the addition of a sequence of numbers, called ''addends'' or ''summands''; the result is their ''sum'' or ''total''. Beside numbers, other types of values can be summed as well: functions, vectors, matrices, polynomials and, in general, elements of any type of mathematical objects on which an operation denoted "+" is defined. Summations of infinite sequences are called series. They involve the concept of limit, and are not considered in this article. The summation of an explicit sequence is denoted as a succession of additions. For example, summation of is denoted , and results in 9, that is, . Because addition is associative and commutative, there is no need for parentheses, and the result is the same irrespective of the order of the summands. Summation of a sequence of only one summand results in the summand itself. Summation of an empty sequence (a sequence with no elements), by convention, results in 0. Very often, the elements of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Limit
In statistical mechanics, the thermodynamic limit or macroscopic limit, of a system is the Limit (mathematics), limit for a large number of particles (e.g., atoms or molecules) where the volume is taken to grow in proportion with the number of particles.S.J. Blundell and K.M. Blundell, "Concepts in Thermal Physics", Oxford University Press (2009) The thermodynamic limit is defined as the limit of a system with a large volume, with the particle density held fixed: : N \to \infty,\, V \to \infty,\, \frac N V =\text In this limit, macroscopic thermodynamics is valid. There, thermal fluctuations in global quantities are negligible, and all List of thermodynamic properties, thermodynamic quantities, such as pressure and energy, are simply functions of the thermodynamic variables, such as temperature and density. For example, for a large volume of gas, the fluctuations of the total internal energy are negligible and can be ignored, and the average internal energy can be predicted fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann's Distribution
In statistical mechanics and mathematics, a Boltzmann distribution (also called Gibbs distribution Translated by J.B. Sykes and M.J. Kearsley. See section 28) is a probability distribution or probability measure that gives the probability that a system will be in a certain microstate (statistical mechanics), state as a function of that state's energy and the temperature of the system. The distribution is expressed in the form: :p_i \propto \exp\left(- \frac \right) where is the probability of the system being in state , is the exponential function, is the energy of that state, and a constant of the distribution is the product of the Boltzmann constant and thermodynamic temperature . The symbol \propto denotes proportionality (mathematics), proportionality (see for the proportionality constant). The term ''system'' here has a wide meaning; it can range from a collection of 'sufficient number' of atoms or a single atom to a macroscopic system such as a Natural gas storag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |