|
Embonate
Pamoic acid, also called embonic acid, is a 2-Naphthoic acid derivative. Salts and esters of pamoic acid are known as pamoates or embonates. It can be prepared by the reaction of 3-hydroxy-2-naphthoic acid with formaldehyde. In pharmacology, the salt form of pamoic acid (pamoate ion) can be used as a counterion of a drug compound to affect the dissolution rate of the drug. The presence of multiple oxygen atoms enables significant hydrogen bonding to occur. Hydrogen bonds facilitate the dissolution of compounds in water. Pharmaceutical drugs formulated this way include cycloguanil pamoate, hydroxyzine pamoate, imipramine pamoate, olanzapine pamoate hydrate, oxantel pamoate, pyrantel pamoate, and pyrvinium pamoate. Pamoic acid has agonist activity for the orphan G protein-coupled receptor G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imipramine Pamoate
Imipramine, sold under the brand name Tofranil, among others, is a tricyclic antidepressant (TCA) mainly used in the treatment of depression (mood), depression. It is also effective in treating anxiety and panic disorder. Imipramine is taken oral administration, by mouth. Common side effects of imipramine include dry mouth, drowsiness, dizziness, hypotension, low blood pressure, tachycardia, rapid heart rate, urinary retention, and electrocardiogram changes. Overdose of the medication can result in death. Imipramine appears to work by Serotonin–norepinephrine reuptake inhibitor, increasing levels of serotonin and norepinephrine and by receptor antagonist, blocking certain serotonin, adrenergic receptor, adrenergic, histamine receptor, histamine, and acetylcholine receptor, cholinergic receptor (biochemistry), receptors. Imipramine was discovered in 1951 and was introduced for medical use in 1957. It was the first TCA to be marketed. Imipramine and TCAs other than amitriptyline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrvinium Pamoate
Pyrvinium (Viprynium) is an anthelmintic effective for pinworms. Several forms of pyrvinium have been prepared with variable counter anions, such as halides, tosylate, triflate and pamoate Pamoic acid, also called embonic acid, is a 2-Naphthoic acid derivative. Salts and esters of pamoic acid are known as pamoates or embonates. It can be prepared by the reaction of 3-hydroxy-2-naphthoic acid with formaldehyde. In pharmacology .... Pyrvinium was identified as a potent Wnt inhibitor, acting through activation of Casein kinase CK1α. Pyrvinium salts can also inhibit the growth of cancer cells. More specifically, the pamoate salt has been shown to have preferential toxicity for various cancer cell lines during glucose starvation. Synthesis One synthetic method is based on Skraup synthesis and Paal-Knorr synthesis. More recently, an alternative convergent, synthetic strategy to pyrvinium triflate salts through Friedländer synthesis was reported. References An ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyzine Pamoate
Hydroxyzine, sold under the brand names Atarax and Vistaril among others, is an antihistamine medication. It is used in the treatment of itchiness, anxiety, insomnia, and nausea (including that due to motion sickness). It is used either by mouth or injection into a muscle. Hydroxyzine works by blocking the effects of histamine. It is a first-generation antihistamine in the piperazine family of chemicals. Common side effects include sleepiness, headache, and dry mouth. Serious side effects may include QT prolongation. It is unclear if use during pregnancy or breastfeeding is safe. It was first made by Union Chimique Belge in 1956 and was approved for sale by Pfizer in the United States later that year. In 2022, it was the 46th most commonly prescribed medication in the United States, with more than 13million prescriptions. Medical uses Hydroxyzine is used in the treatment of itchiness, anxiety, and nausea due to motion sickness. A systematic review concluded that hydroxyz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Merck Index
''The Merck Index'' is an encyclopedia of chemical substance, chemicals, pharmaceutical drug, drugs and biomolecule, biologicals with over 10,000 monographs on single substances or groups of related chemical compound, compounds published online by the Royal Society of Chemistry. History The first edition of the Merck's Index was published in 1889 by the German chemical company Merck Group, Emanuel Merck and was primarily used as a sales catalog for Merck's growing list of chemicals it sold. The American subsidiary was established two years later and continued to publish it. During World War I the US government seized Merck's US operations and made it a separate American "Merck" company that continued to publish the Merck Index. In 2012 the Merck Index was licensed to the Royal Society of Chemistry. An online version of The Merck Index, including historic records and new updates not in the print edition, is commonly available through research libraries. It also includes an append ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
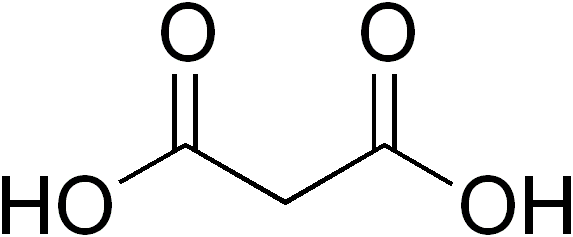
Dicarboxylic Acids
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or Aromatic compound, aromatic.Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. In general, dicarboxylic acids show similar chemical behavior and reactivity to carboxylic acid, monocarboxylic acids. Dicarboxylic acids are usually colorless solids. A wide variety of dicarboxylic acids are used in industry. Adipic acid, for example, is a precursor to certain kinds of nylon. A wide variety of dicarboxylic acids are found in nature. Aspartic acid and glutamic acid are two amino acids found in all life. Succinic and fumaric acids are essential for metabolism. A large inventory of derivatives are known including many mono- and diesters, amides, etc. Partial list of saturated dicarboxylic acids Some common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antinociceptive
In physiology, nociception , also nocioception; ) is the sensory nervous system's process of encoding noxious stimuli. It deals with a series of events and processes required for an organism to receive a painful stimulus, convert it to a molecular signal, and recognize and characterize the signal to trigger an appropriate defensive response. In nociception, intense chemical (e.g., capsaicin present in chili pepper or cayenne pepper), mechanical (e.g., cutting, crushing), or thermal (heat and cold) stimulation of sensory neurons called nociceptors produces a signal that travels along a chain of nerve fibers to the brain. Nociception triggers a variety of physiological and behavioral responses to protect the organism against an aggression, and usually results in a subjective experience, or perception, of pain in sentient beings. Detection of noxious stimuli Potentially damaging mechanical, thermal, and chemical stimuli are detected by nerve endings called nociceptors, which are f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arrestin Beta 2
Beta-arrestin-2, or β-arrestin2, also known as arrestin beta-2, is an intracellular protein that in humans is encoded by the ''ARRB2'' gene. Members of arrestin/beta-arrestin protein family are thought to participate in agonist-mediated desensitization of G protein-coupled receptors and cause specific dampening of cellular responses to stimuli such as hormones, neurotransmitters, or sensory signals, as well as having signalling roles in their own right. Arrestin beta 2, like arrestin beta 1, was shown to inhibit beta-adrenergic receptor function in vitro. It is expressed at high levels in the central nervous system and may play a role in the regulation of synaptic receptors. Besides the brain, a cDNA for arrestin beta 2 was isolated from thyroid gland, and thus it may also be involved in hormone-specific desensitization of TSH receptors. Multiple alternatively spliced transcript variants have been found for this gene, but the full-length nature of some variants has not been de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extracellular Signal-regulated Kinases
This glossary of biology terms is a list of definitions of fundamental terms and concepts used in biology, the study of life and of living organisms. It is intended as introductory material for novices; for more specific and technical definitions from sub-disciplines and related fields, see Glossary of cell biology, Glossary of genetics, Glossary of evolutionary biology, Glossary of ecology, Glossary of environmental science and Glossary of scientific naming, or any of the organism-specific glossaries in :Glossaries of biology. A B C D E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GPR35
G protein-coupled receptor 35 also known as GPR35 is a G protein-coupled receptor which in humans is encoded by the ''GPR35'' gene. Heightened expression of GPR35 is found in immune and gastrointestinal tissues, including the crypts of Lieberkühn. Ligands Endogenous ligands Although GPR35 is still considered an orphan receptor, there have been attempts to deorphanize it by identifying endogenous molecules that can activate the receptor. All of the currently proposed ligands are either unselective towards GPR35, or they lack high potency, a characteristic feature of natural ligands. The following list includes the most prominent examples: * kynurenic acidfree fulltext * LPA species [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein-coupled Receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in the form of six loops (three extracellular loops interacting with ligand molecules, three intracellular loops interacting with G proteins, an N-terminal extracellular region and a C-terminal intracellular region) of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Text was copied from this source, which is available under Attribution 2.5 Generic (CC BY 2.5) licence/ref> Ligands can bind either to the extracellular N-terminus and loops (e.g. glutamate receptors) or to the binding site wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |