|
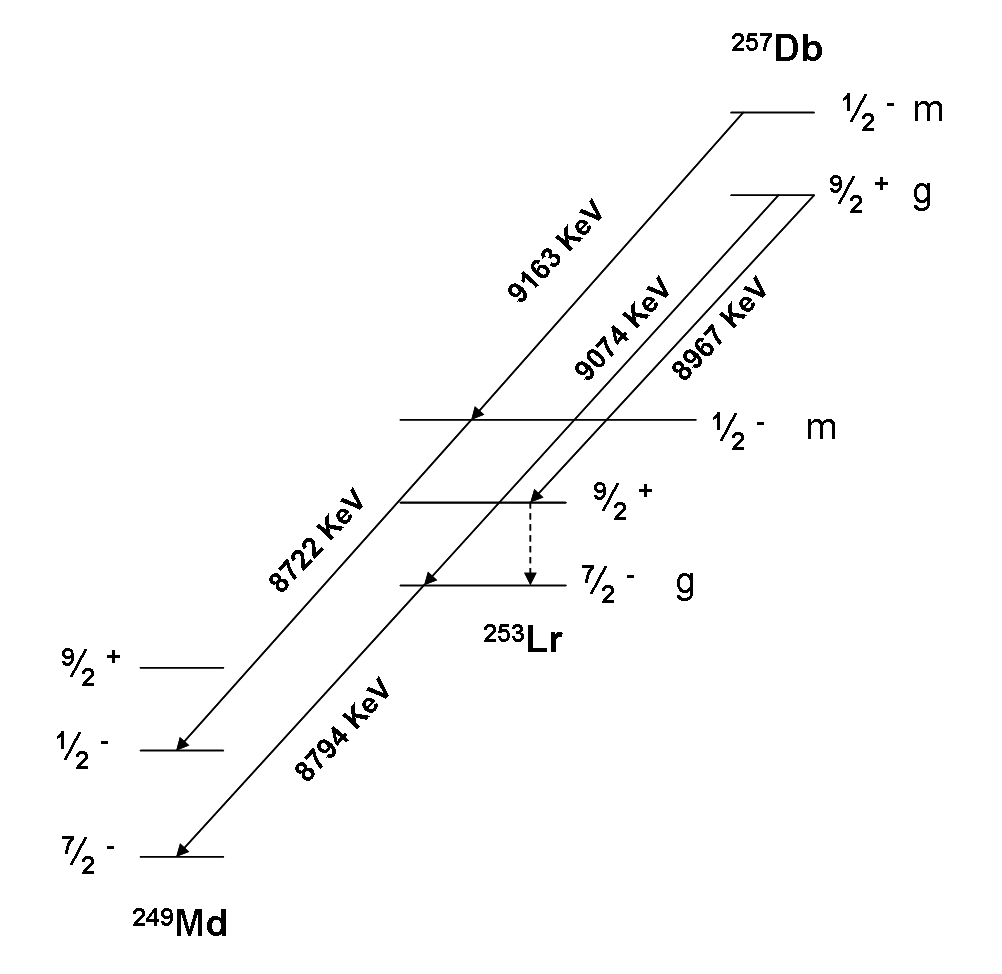
Dubnium-257
Dubnium (105Db) is a synthetic element, thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 261Db in 1968. Thirteen radioisotopes are known, ranging from 255Db to 270Db (except 264Db, 265Db, and 269Db), along with one isomer (257mDb); two more isomers have been reported but are unconfirmed. The longest-lived known isotope is 268Db with a half-life of 16 hours. List of isotopes , -id=Dubnium-255 , rowspan=2, 255Db , rowspan=2 style="text-align:right" , 105 , rowspan=2 style="text-align:right" , 150 , rowspan=2, 255.10692(30)# , rowspan=2, 54 ms , SF (67%) , (various) , rowspan=2, 9/2+# , - , α (33%?) , 251Lr , -id=Dubnium-255m , rowspan=2, 255mDbOrder of ground state and isomer is uncertain. , rowspan=2 colspan=3 style="text-indent:2em", 100(100)# keV , rowspan=2, , SF (92%) , (various) , rowspan=2, 1/2−# , - , α (8%) , 251Lr , -id=Dubnium-256 , rowspan=2, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dubnium
Dubnium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Db and atomic number 105. It is highly radioactive: the most stable known isotopes of dubnium, isotope, dubnium-268, has a half-life of about 16 hours. This greatly limits extended research on the element. Dubnium does not occur naturally on Earth and is produced artificially. The Soviet Joint Institute for Nuclear Research (JINR) claimed the first discovery of the element in 1968, followed by the American Lawrence Berkeley Laboratory in 1970. Both teams proposed their names for the new element and used them without formal approval. The long-standing dispute was resolved in 1993 by an official investigation of the discovery claims by the Transfermium Working Group, formed by the International Union of Pure and Applied Chemistry and the International Union of Pure and Applied Physics, resulting in credit for the discovery being officially shared between both teams. The element was formally ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in what is called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron emission to be energeticall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lawrencium
Lawrencium is a synthetic chemical element; it has symbol Lr (formerly Lw) and atomic number 103. It is named after Ernest Lawrence, inventor of the cyclotron, a device that was used to discover many artificial radioactive elements. A radioactive metal, lawrencium is the eleventh transuranium element, the third transfermium, and the last member of the actinide series. Like all elements with atomic number over 100, lawrencium can only be produced in particle accelerators by bombarding lighter elements with charged particles. Fourteen isotopes of lawrencium are currently known; the most stable is 266Lr with half-life 11 hours, but the shorter-lived 260Lr (half-life 2.7 minutes) is most commonly used in chemistry because it can be produced on a larger scale. Chemistry experiments confirm that lawrencium behaves as a heavier homolog to lutetium in the periodic table, and is a trivalent element. It thus could also be classified as the first of the 7th-period transition metals. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rutherfordium
Rutherfordium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Rf and atomic number 104. It is named after physicist Ernest Rutherford. As a synthetic element, it is not found in nature and can only be made in a particle accelerator. It is radioactive; the most stable known isotope, 267Rf, has a half-life of about 48 minutes. In the periodic table, it is a d-block element and the second of the fourth-row transition elements. It is in period 7 and is a group 4 element. Chemistry experiments have confirmed that rutherfordium behaves as the heavier Homologous series, homolog to hafnium in group 4. The chemical properties of rutherfordium are characterized only partly. They compare well with the other group 4 elements, even though some calculations had indicated that the element might show significantly different properties due to Relativistic quantum chemistry, relativistic effects. In the 1960s, small amounts of rutherfordium were produced at Joint ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Jyväskylä
A university () is an institution of tertiary education and research which awards academic degrees in several academic disciplines. ''University'' is derived from the Latin phrase , which roughly means "community of teachers and scholars". Universities typically offer both undergraduate and postgraduate programs. The first universities in Europe were established by Catholic monks. The University of Bologna (), Italy, which was founded in 1088, is the first university in the sense of: *being a high degree-awarding institute. *using the word (which was coined at its foundation). *having independence from the ecclesiastic schools and issuing secular as well as non-secular degrees (with teaching conducted by both clergy and non-clergy): grammar, rhetoric, logic, theology, canon law and notarial law.Hunt Janin: "The university in medieval life, 1179–1499", McFarland, 2008, , p. 55f.de Ridder-Symoens, Hilde''A History of the University in Europe: Volume 1, Universities in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Einsteinium
Einsteinium is a synthetic chemical element; it has symbol Es and atomic number 99 and is a member of the actinide series and the seventh transuranium element. Einsteinium was discovered as a component of the debris of the first hydrogen bomb explosion in 1952. Its most common isotope, einsteinium-253 (Es; half-life 20.47 days), is produced artificially from decay of californium-253 in a few dedicated high-power nuclear reactors with a total yield on the order of one milligram per year. The reactor synthesis is followed by a complex process of separating einsteinium-253 from other actinides and products of their decay. Other isotopes are synthesized in various laboratories, but in much smaller amounts, by bombarding heavy actinide elements with light ions. Due to the small amounts of einsteinium produced and the short half-life of its most common isotope, there are no practical applications for it except basic scientific research. In particular, einsteinium was used to synthesiz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mendelevium
Mendelevium is a synthetic chemical element; it has symbol Md ( formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced in macroscopic quantities by neutron bombardment of lighter elements. It is the third-to-last actinide and the ninth transuranic element and the first transfermium. It can only be produced in particle accelerators by bombarding lighter elements with charged particles. Seventeen isotopes are known; the most stable is 258Md with half-life 51.59 days; however, the shorter-lived 256Md (half-life 77.7 minutes) is most commonly used in chemistry because it can be produced on a larger scale. Mendelevium was discovered by bombarding einsteinium with alpha particles in 1955, the method still used to produce it today. It is named after Dmitri Mendeleev, the father of the periodic table. Using available microgram quantities of e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spontaneous Fission
Spontaneous fission (SF) is a form of radioactive decay in which a heavy atomic nucleus splits into two or more lighter nuclei. In contrast to induced fission, there is no inciting particle to trigger the decay; it is a purely probabilistic process. Spontaneous fission is a dominant decay mode for superheavy elements, with nuclear stability generally falling as nuclear mass increases. It thus forms a practical limit to heavy element nucleon number. Heavier nuclides may be created instantaneously by physical processes, both natural (via the r-process, ''r''-process) and artificial, though rapidly decay to more stable nuclides. As such, apart from minor decay branches in primordial radionuclides, spontaneous fission is not observed in nature. Observed fission half-lives range from 60 nanoseconds () to greater than the current age of the universe (). History Following the discovery of induced fission by Otto Hahn and Fritz Strassmann in 1938, Soviet physicists Georgy Flyorov and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Chain
In nuclear science a decay chain refers to the predictable series of radioactive disintegrations undergone by the nuclei of certain unstable chemical elements. Radioactive isotopes do not usually decay directly to stable isotopes, but rather into another radioisotope. The isotope produced by this radioactive emission then decays into another, often radioactive isotope. This chain of decays always terminates in a stable isotope, whose nucleus no longer has the surplus of energy necessary to produce another emission of radiation. Such stable isotopes may be said to have reached their '' ground states''. The stages or steps in a decay chain are referred to by their relationship to previous or subsequent stages. Hence, a ''parent isotope'' is one that undergoes decay to form a ''daughter isotope''. For example element 92, uranium, has an isotope with 144 neutrons ( 236U) and it decays into an isotope of element 90, thorium, with 142 neutrons ( 232Th). The daughter isotope may be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phys
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which relates to the order of nature, or, in other words, to the regular succession of events." It is one of the most fundamental scientific disciplines. "Physics is one of the most fundamental of the sciences. Scientists of all disciplines use the ideas of physics, including chemists who study the structure of molecules, paleontologists who try to reconstruct how dinosaurs walked, and climatologists who study how human activities affect the atmosphere and oceans. Physics is also the foundation of all engineering and technology. No engineer could design a flat-screen TV, an interplanetary spacecraft, or even a better mousetrap without first understanding the basic laws of physics. (...) You will come to see physics as a towering achievement of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Element
A synthetic element is a known chemical element that does not occur naturally on Earth: it has been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, it is called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table: these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons into the Atomic nucleus, nucleus of an element with an atomic number lower than 95. All known (see: Island of stability) synthetic elements are unstable, but they radioactive decay, decay at widely varying rates; the half-lives of their longest-lived isotopes range from microseconds to millions of years. Five more elements that were first created artificially are strictly speaking not ''synthetic'' because they were later found in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |