|
Bone Seeker
A bone seeker is an element, often a radioisotope, that tends to accumulate in the bones of humans and other animals when introduced into the body. For example, strontium and radium are chemically similar to calcium and can replace the calcium in bones. Plutonium is also a bone seeker, though the mechanism by which it accumulates in bone tissue is unknown. Radioactive bone seekers are particular health risks as they irradiate surrounding tissue, though this can be useful for radiotherapy, such as in the case of radium-223. Stable bone seekers can also be harmful: excessive strontium absorption has been linked with increased levels of rickets. The salt strontium ranelate, however, is a bone seeker which is sometimes used to strengthen bones as a treatment for osteoporosis. Bone seekers have been proposed as a method of delivering antibiotic An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemical Element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its atomic nucleus, nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements form Homonuclear molecule, molecules of atoms of said element only: e.g. atoms of hydrogen (H) form Diatomic molecule, diatomic molecules (H). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ionizing Radiation
Ionizing (ionising) radiation, including Radioactive decay, nuclear radiation, consists of subatomic particles or electromagnetic waves that have enough energy per individual photon or particle to ionization, ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum. Gamma rays, X-rays, and the higher energy vacuum ultraviolet, ultraviolet part of the electromagnetic spectrum are ionizing radiation; whereas the lower energy ultraviolet, visible light, infrared, microwaves, and radio waves are non-ionizing radiation. Nearly all types of laser light are non-ionizing radiation. The boundary between ionizing and non-ionizing radiation in the ultraviolet area cannot be sharply defined, as different molecules and atoms ionize at Ionization energies of the elements (data page), different energies. The energy of ionizing radiation starts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
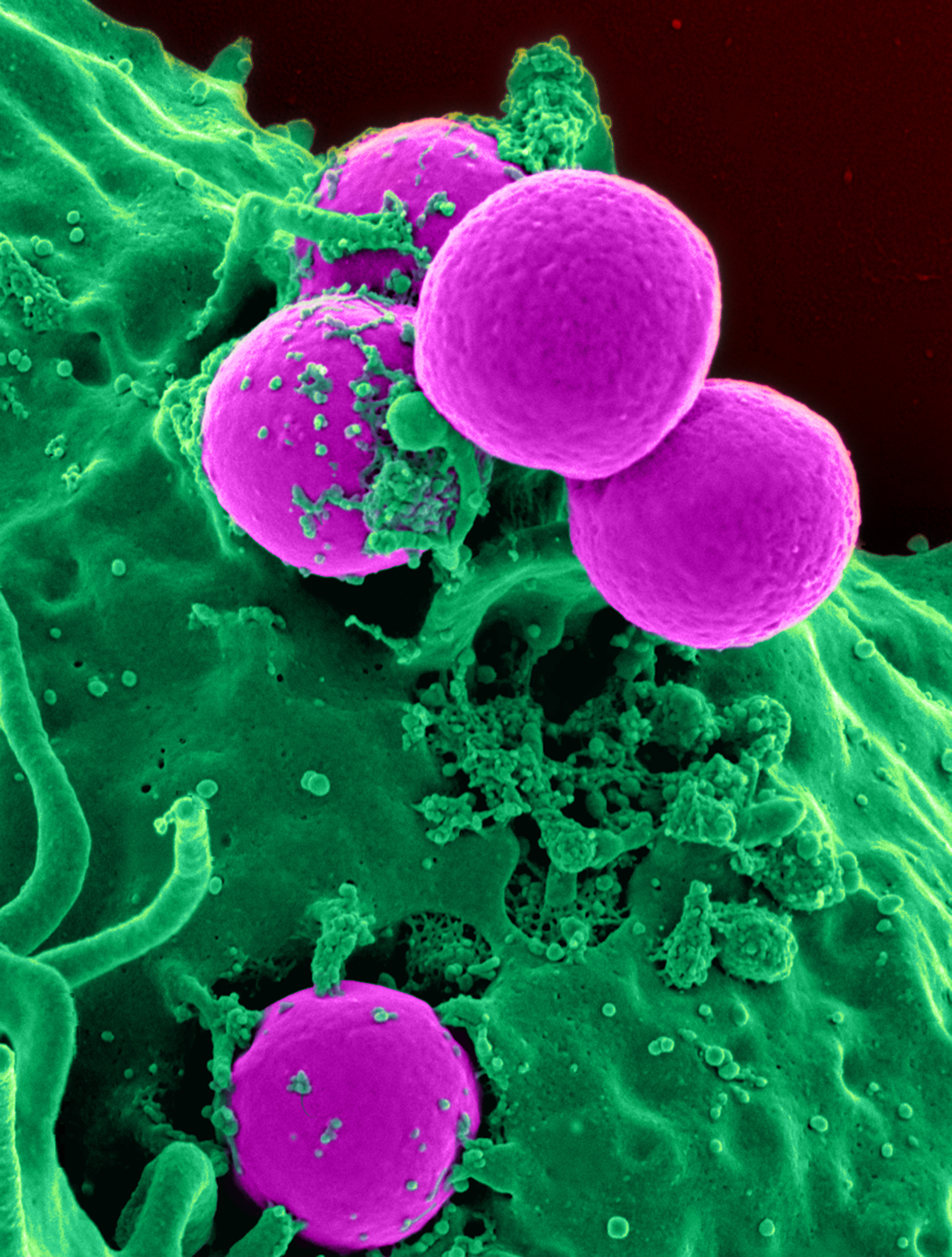
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, treatment and antibiotic prophylaxis, prevention of such infections. They may either bactericide, kill or bacteriostatic agent, inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza. Drugs which inhibit growth of viruses are termed antiviral drugs or antivirals. Antibiotics are also not effective against fungi. Drugs which inhibit growth of fungi are called antifungal drugs. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek language, Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
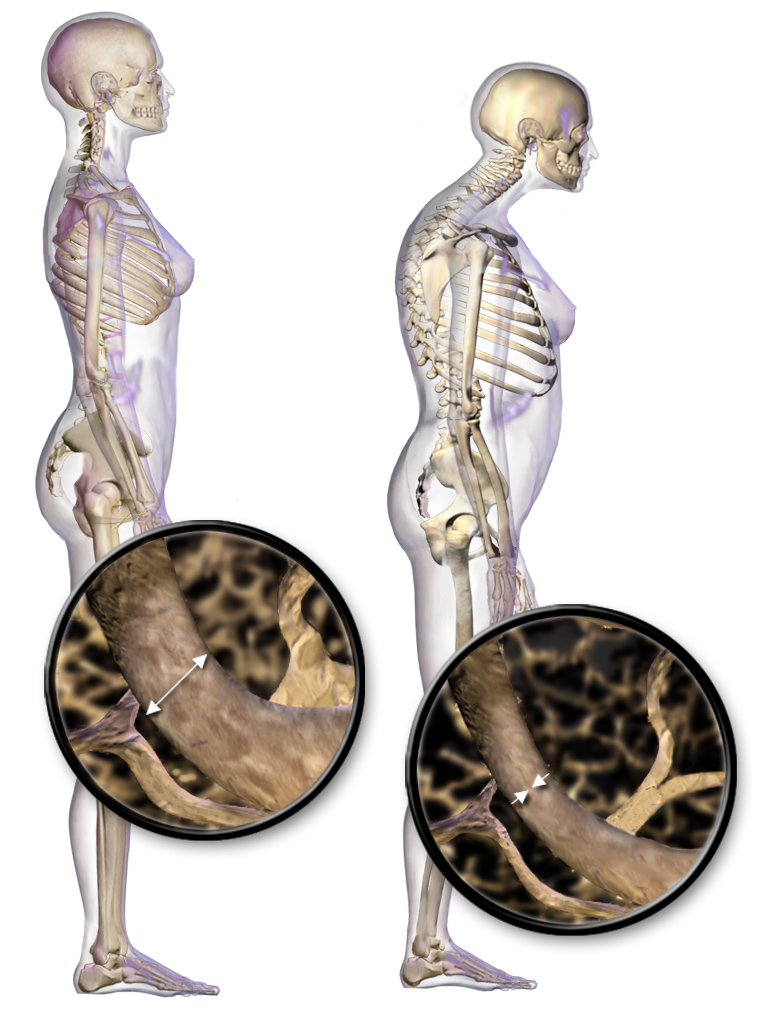
Osteoporosis
Osteoporosis is a systemic skeletal disorder characterized by low bone mass, micro-architectural deterioration of bone tissue leading to more porous bone, and consequent increase in Bone fracture, fracture risk. It is the most common reason for a broken bone among the Old age, elderly. Bones that commonly break include the vertebrae in the Vertebral column, spine, the bones of the forearm, the wrist, and the hip. Until a broken bone occurs there are typically no symptoms. Bones may weaken to such a degree that a break may occur with minor stress or spontaneously. After the broken bone heals, some people may have chronic pain and a decreased ability to carry out normal activities. Osteoporosis may be due to lower-than-normal peak bone mass, maximum bone mass and greater-than-normal bone loss. Bone loss increases after menopause in women due to lower levels of estrogen, and after andropause in older men due to lower levels of testosterone. Osteoporosis may also occur due to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Strontium Ranelate
Strontium ranelate, a strontium(II) salt of ranelic acid, is a medication for osteoporosis marketed as Protelos or Protos by Servier. Studies indicate it can also slow the course of osteoarthritis of the knee. The drug is unusual in that it both increases deposition of new bone by osteoblasts and reduces the resorption of bone by osteoclasts. It is therefore promoted as a "dual action bone agent" (DABA). On 13 May 2013, Servier released a Direct Healthcare Professional Communication which stated that new restrictions for the use of strontium ranelate are now in place, as randomised trials have shown an increased risk of myocardial infarction. Servier states that the use is now restricted to treatment of severe osteoporosis in postmenopausal women at high risk for fracture. The European Pharmacovigilance Risk Assessment Committee (PRAC) recommended restriction in the use of strontium ranelate, based on a routine benefit-risk assessment of the medicine, which included data showin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Rickets
Rickets, scientific nomenclature: rachitis (from Greek , meaning 'in or of the spine'), is a condition that results in weak or soft bones in children and may have either dietary deficiency or genetic causes. Symptoms include bowed legs, stunted growth, bone pain, large forehead, and trouble sleeping. Complications may include bone Deformity, deformities, bone pseudofractures and Bone fracture, fractures, muscle spasms, or an scoliosis, abnormally curved spine. The analogous condition in adults is osteomalacia. The most common cause of rickets is a hypovitaminosis D, vitamin D deficiency, although hereditary genetic forms also exist. This can result from eating a diet without enough vitamin D, dark skin, too little sun exposure, exclusive breastfeeding without vitamin D supplementation, celiac disease, and certain genetic conditions. Other factors may include not enough calcium or phosphorus. The underlying mechanism involves insufficient calcification of the growth plate. Di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Radium-223
Radium-223 (223Ra, Ra-223) is an Isotopes of radium#Radium-223, isotope of radium with an 11.4-day half-life. It was discovered in 1905 by T. Godlewski, a Polish chemist from Kraków, and was historically known as Decay chain#Actinium series, actinium X (AcX). Radium-223 dichloride is an alpha particle-emitting radiotherapy drug that mimics calcium and forms complexes with hydroxyapatite at areas of increased bone turnover. The principal use of radium-223, as a radiopharmaceutical to treat Metastasis, metastatic cancers in bone, takes advantage of its chemical similarity to calcium, and the short range of the alpha radiation it emits. Origin and preparation Although radium-223 is naturally formed in trace amounts by the Decay chain#Actinium series, decay of uranium-235, it is generally made artificially,Bruland O.S., Larsen R.H. (2003). Radium revisited. In: Bruland O.S., Flgstad T., editors. Targeted cancer therapies: An odyssey. University Library of Tromso, Ravnetrykk No. 29. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
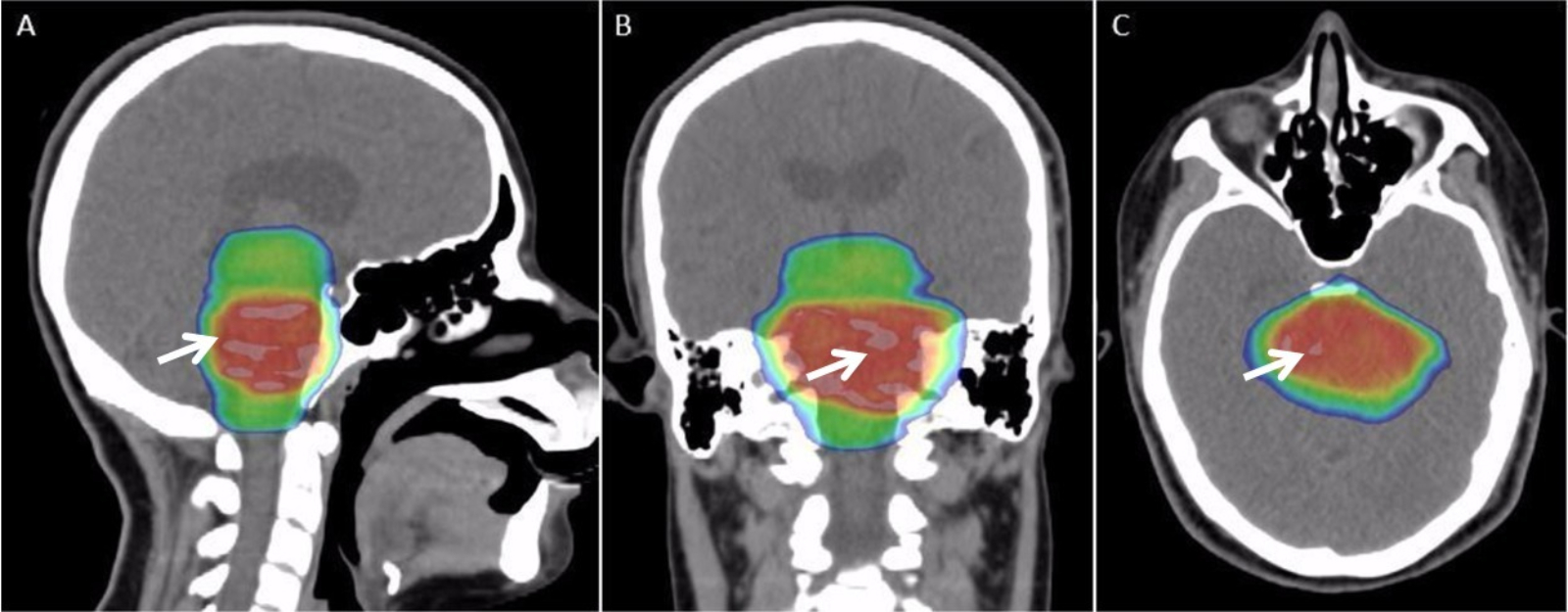
Radiotherapy
Radiation therapy or radiotherapy (RT, RTx, or XRT) is a treatment using ionizing radiation, generally provided as part of cancer therapy to either kill or control the growth of malignant cells. It is normally delivered by a linear particle accelerator. Radiation therapy may be curative in a number of types of cancer if they are localized to one area of the body, and have not spread to other parts. It may also be used as part of adjuvant therapy, to prevent tumor recurrence after surgery to remove a primary malignant tumor (for example, early stages of breast cancer). Radiation therapy is synergistic with chemotherapy, and has been used before, during, and after chemotherapy in susceptible cancers. The subspecialty of oncology concerned with radiotherapy is called radiation oncology. A physician who practices in this subspecialty is a radiation oncologist. Radiation therapy is commonly applied to the cancerous tumor because of its ability to control cell growth. Ionizin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Radioactive Decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered ''radioactive''. Three of the most common types of decay are Alpha decay, alpha, Beta decay, beta, and Gamma ray, gamma decay. The weak force is the Fundamental interactions, mechanism that is responsible for beta decay, while the other two are governed by the electromagnetic force, electromagnetic and nuclear forces. Radioactive decay is a randomness, random process at the level of single atoms. According to quantum mechanics, quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant or as a half-life. The half-lives of radioactive atoms have a huge range: f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation states. It reacts with carbon, halogens, nitrogen, silicon, and hydrogen. When exposed to moist air, it forms oxides and hydrides that can expand the sample up to 70% in volume, which in turn flake off as a powder that is pyrophoric. It is radioactive and can accumulate in bones, which makes the handling of plutonium dangerous. Plutonium was first synthesized and isolated in late 1940 and early 1941, by deuteron bombardment of uranium-238 in the cyclotron at the University of California, Berkeley. First, neptunium-238 (half-life 2.1 days) was synthesized, which then beta-decayed to form the new element with atomic number 94 and atomic weight 238 (half-life 88 years). Since uranium had been named after the planet Uranus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossils of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name comes from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceuticals for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |