|
Alpha-Ethylphenethylamine
Phenylisobutylamine, also known as α-ethylphenethylamine (AEPEA) or as butanphenamine (B), is a stimulant drug of the phenethylamine and amphetamine families. It is a higher homologue of amphetamine, differing from amphetamine's molecular structure only by the substitution of the methyl group at the alpha position of the side chain with an ethyl group. Phenylisobutylamine acts as a norepinephrine–dopamine releasing agent (NDRA) and has been found to produce stimulant-like and reinforcing effects in animals. It shows much lower potency and a greater preference for induction of norepinephrine release compared to dextroamphetamine. "Phenylisobutylamine" is in fact a chemical misnomer because isobutylamine itself contains a branched chain. The correct name after this style for this class of compound would be "phenyl''sec''butylamine". Derivatives A number of notable derivatives of phenylisobutylamine are known, including the following: Additional derivatives with longer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzodioxolylbutanamine
1,3-Benzodioxolylbutanamine (also known as 3,4-methylenedioxybutanphenamine, MDB, BDB, J, and 3,4-methylenedioxy-α-ethylphenethylamine) is an entactogenic drug of the phenethylamine, amphetamine, and phenylisobutylamine families. It is the α- ethyl analog of MDPEA and MDA and the methylenedioxy analogue of α-ethylphenethylamine. BDB was first synthesized by Alexander Shulgin. In his book ''PiHKAL'', the dosage range is listed as 150–230 mg and the duration is listed as 4–8 hours. BDB produces entactogenic, MDMA-like effects. Although pleasant and euphoric, BDB is also fairly sedating and some users feel that the lack of stimulant effect makes it less enjoyable than other similar drugs. Additional side effects associated with BDB include nystagmus and dizziness. Very little data exists about the pharmacological properties, metabolism, and toxicity of BDB. Receptor and transporter interaction data have been reported for BDB. Animal studies and anecdotal reports show ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Schedule II Drugs
A list is a set of discrete items of information collected and set forth in some format for utility, entertainment, or other purposes. A list may be memorialized in any number of ways, including existing only in the mind of the list-maker, but lists are frequently written down on paper, or maintained electronically. Lists are "most frequently a tool", and "one does not ''read'' but only ''uses'' a list: one looks up the relevant information in it, but usually does not need to deal with it as a whole". Lucie Doležalová,The Potential and Limitations of Studying Lists, in Lucie Doležalová, ed., ''The Charm of a List: From the Sumerians to Computerised Data Processing'' (2009). Purpose It has been observed that, with a few exceptions, "the scholarship on lists remains fragmented". David Wallechinsky, a co-author of '' The Book of Lists'', described the attraction of lists as being "because we live in an era of overstimulation, especially in terms of information, and lists help ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
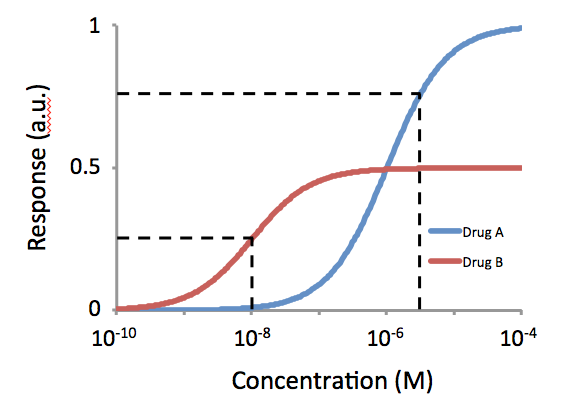
Potency (pharmacology)
In pharmacology, potency or biological potency is a measure of a drug's biological activity expressed in terms of the dose required to produce a pharmacological effect of given intensity. A highly potent drug (e.g., fentanyl, clonazepam, risperidone, benperidol, bumetanide) evokes a given response at low concentrations, while a drug of lower potency (e.g. morphine, alprazolam, ziprasidone, haloperidol, furosemide) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness nor more side effects nor less side effects. Types of potency The International Union of Basic and Clinical Pharmacology (IUPHAR) has stated that "potency is an imprecise term that should always be further defined", and lists of types of potency as follows: Miscellaneous Lysergic acid diethylamide (LSD) is one of the most potent psychoactive drug A psychoactive drug, psychopharmaceutical, mind-altering drug, consciousness-altering drug, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Methylphenylisobutylamine
4-Methylphenylisobutylamine (4-MAB), also known as 4-methyl-α-ethylphenethylamine, is a stimulant drug of the phenethylamine, amphetamine, and phenylisobutylamine families. See also * Phenylisobutylamine * 4-Methylamphetamine * Benzodioxolylbutanamine 1,3-Benzodioxolylbutanamine (also known as 3,4-methylenedioxybutanphenamine, MDB, BDB, J, and 3,4-methylenedioxy-α-ethylphenethylamine) is an entactogenic drug of the phenethylamine, amphetamine, and phenylisobutylamine families. It is the α- ... References {{DEFAULTSORT:Methylphenylisobutylamine, 4- Phenylisobutylamines Serotonin-norepinephrine-dopamine releasing agents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
6-MBPB
6-MBPB, also known as 6-(2-methylaminobutyl)benzofuran (6-MABB), is a monoamine releasing agent (MRA) and entactogen-like drug of the substituted amphetamine, amphetamine, phenylisobutylamine, and substituted benzofuran, benzofuran families. It is a positional isomer of 5-MBPB (5-MABB). The drug appears to act as a serotonin–norepinephrine–dopamine releasing agent (SNDRA). The values for induction of monoamine release in rat brain synaptosomes have been reported for the individual enantiomers of 6-MBPB. In the case of (''S'')-6-MBPB, they were 54nM for serotonin, 77nM for norepinephrine, and 41nM for dopamine, whereas for (''R'')-6-MBPB, they were 172nM for serotonin, 227nM for norepinephrine, and inactive for dopamine. Hence, (''S'')-6-MBPB is an SNDRA, whereas (''R'')-MBPB is a serotonin–norepinephrine releasing agent (SNRA). The enantiomers showed a mixed profile of acting as full versus partial monoamine releaser, partial releasers. 6-MBPB partially substituted for MDMA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-MBPB
5-MBPB (also known as 5-MPBP and 5-MABB) is an amphetamine and phenylisobutylamine derivative which is structurally related to MDMA and has been sold as a designer drug. It can be described as the benzofuran-5-yl analogue of MBDB or the butanamine homologue of 5-MAPB, and is also a structural isomer of 5-EAPB and 6-EAPB. Anecdotal reports suggest this compound has been sold as a designer drug in various European countries since early 2015, but the first definitive identification was made in December 2015 by a forensic laboratory in Slovenia. 5-MBPB is similar in structure to compounds such as 5-APB which are claimed to be agonists of the 5-HT2C receptor. 5-MBPB (5-MABB) has been found to act as a potent serotonin–norepinephrine–dopamine releasing agent (SNDRA), with preference for induction of serotonin release over norepinephrine and dopamine release, and fully substitutes for MDMA in animal drug discrimination tests. See also * 6-MBPB 6-MBPB, also known as 6-(2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylbenzodioxolylbutanamine
Ethylbenzodioxolylbutanamine (EBDB; Ethyl-J) is a lesser-known entactogen, stimulant, and psychedelic drug, psychedelic of the substituted phenethylamine, phenethylamine, substituted amphetamine, amphetamine, and phenylisobutylamine families. It is the ''N''-ethyl group, ethyl analogue (chemistry), analogue of benzodioxylbutanamine (BDB; "J"), and also the alpha carbon, α-ethyl group, ethyl analogue of methylenedioxyethylamphetamine (MDEA; "Eve"). EBDB was first synthesized by Alexander Shulgin. In his book ''PiHKAL'', the minimum dosage consumed was 90 milligram, mg, and the duration is unknown. EBDB produced few to no effects at the dosage range tested in ''PiHKAL'', but at higher doses of several hundred milligrams it produces euphoric effects similar to those of methylbenzodioxylbutanamine (MBDB; "Eden", "Methyl-J"), although milder and shorter lasting. Very little data exists about the pharmacological properties, metabolism, and toxicity of EBDB. See also * Methylbenzodio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylbenzodioxolylbutanamine
MBDB, also known as ''N''-methyl-1,3-benzodioxolylbutanamine or as 3,4-methylenedioxy-''N''-methyl-α-ethylphenylethylamine, is an entactogen of the phenethylamine, amphetamine, and phenylisobutylamine families related to MDMA. It is known by the street names "Eden" and "Methyl-J". History and effects MBDB was first synthesized by pharmacologist and medicinal chemist David E. Nichols and later tested by Alexander Shulgin and described in his book, '' PiHKAL: A Chemical Love Story''. MBDB's dosage, according to ''PiHKAL'', is 180 to 210mg; the proper dosage relative to body mass seems unknown. Its duration is 4 to 6hours, with noticeable after-effects lasting for 1 to 3hours. MBDB was initially developed as a non- psychedelic entactogen. It has lower effects on the dopamine system in comparison to other entactogens such as MDMA. MBDB causes many mild, MDMA-like effects, in particular the lowering of social barriers and inhibitions, pronounced sense of empathy and compassion, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N,α-Diethylphenethylamine
''N'',α-Diethylphenethylamine (DEPEA or NADEP), also known as 2-ethylamino-1-phenylbutane (EAPB) is a stimulant drug of the phenylisobutylamine (α-ethylphenethylamine) group. It is a close chemical analog of methamphetamine, which has been sold as a designer drug. It was originally patented by Knoll Pharma as one of several analogs for pharmaceutical applications. In animal models these analogs showed properties of cognitive enhancement and increased pain tolerance. Nevertheless, this class of compounds was never developed into a medicine. Pharmacology DEPEA is a mixed norepinephrine–dopamine releasing agent (NDRA) and norepinephrine–dopamine reuptake inhibitor (NDRI). It is a full releaser of norepinephrine but a weak partial releaser of dopamine with a maximal efficacy of about 40% in rat brain synaptosomes. In another study however, DEPEA non-significantly released norepinephrine but did not release dopamine at all in human embryonic kidney 293 (HEK293) cells transfecte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
α-Ethyl-N-methylphenethylamine
α-Ethyl-''N''-methylphenethylamine (MEPEA; code name PAL-426) is a stimulant, designer drug, and norepinephrine–dopamine releasing agent (NDRA) of the phenethylamine, amphetamine, and phenylisobutylamine (α-ethylphenethylamine) families. It is the ''N''-methyl derivative of phenylisobutylamine (α-ethylphenethylamine; AEPEA) and is the α- ethyl homologue of methamphetamine (α-methyl-''N''-methylphenethylamine). The drug's values for induction of monoamine release are 58nM for norepinephrine, 179 to 225nM for dopamine, and 4,698nM for serotonin. Like amphetamine, MEPEA produces hyperlocomotion and sympathomimetic effects in rodents. It is about one-tenth as potent as ''d''-methamphetamine in drug discrimination and other tests in rodents. MEPEA was first described in the scientific literature by 1984. It has been encountered as an ingredient in dietary supplements. See also * ''N'',α-Diethylphenethylamine * α-Propylphenethylamine * Buphedrone Buphedrone, als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Derivative
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is availa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |