|
Zinc Iodide
Zinc iodide is the inorganic compound with the formula ZnI2. It exists both in anhydrous form and as a dihydrate. Both are white and readily absorb water from the atmosphere. It has no major application. Preparation It can be prepared by the direct reaction of zinc and iodine in water or refluxing ether: : Zn + I2 → ZnI2 Absent a solvent, the elements do not combine directly at room temperature. Structure as solid, gas, and in solution The structure of solid ZnI2 is unusual relative to the dichloride. While zinc centers are tetrahedrally coordinated, as in ZnCl2, groups of four of these tetrahedra share three vertices to form “super-tetrahedra” of composition , which are linked by their vertices to form a three-dimensional structure. These "super-tetrahedra" are similar to the P4O10 structure. Molecular ZnI2 is linear as predicted by VSEPR theory with a Zn-I bond length of 238 pm. In aqueous solution the following have been detected: Zn(H2O)62+, nI(H2O)5sup>+, tetra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure. It was originated by William Burton Pearson and is used extensively in Pearson's handbook of crystallographic data for intermetallic phases. The symbol is made up of two letters followed by a number. For example: * Diamond structure, cF8 * Rutile structure, tP6 Construction The two letters in the Pearson symbol specify the Bravais lattice, and more specifically, the lower-case letter specifies the Crystal system, crystal family, while the upper-case letter the Lattice (group), lattice type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell (atoms which satisfy 1 > x,y,z \geq 0 for the atom's position (x,y,z) in the unit cell). [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
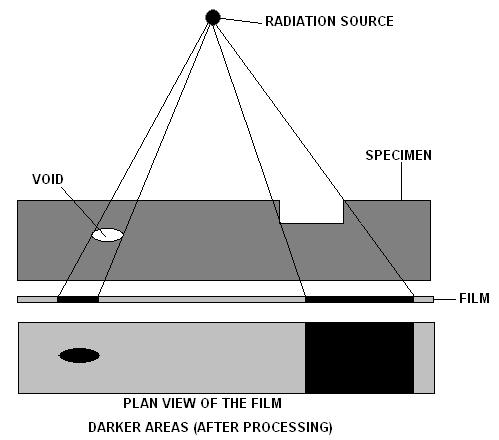
Industrial Radiography
Industrial radiography is a modality of non-destructive testing that uses ionizing radiation to inspect materials and components with the objective of locating and quantifying defects and degradation in material properties that would lead to the failure of engineering structures. It plays an important role in the science and technology needed to ensure product quality and reliability. In Australia, industrial radiographic non-destructive testing is colloquially referred to as "bombing" a component with a "bomb". Industrial Radiography uses either X-rays, produced with X-ray generators, or gamma rays generated by the natural radioactivity of sealed radionuclide sources. Neutrons can also be used. After crossing the specimen, photons are captured by a X-ray detector, detector, such as a silver halide film, a Photostimulated luminescence, phosphor plate, flat panel detector or CdTe detector. The examination can be performed in static 2D (named radiography), in real time 2D (fluorosc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodides
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is not. The low ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
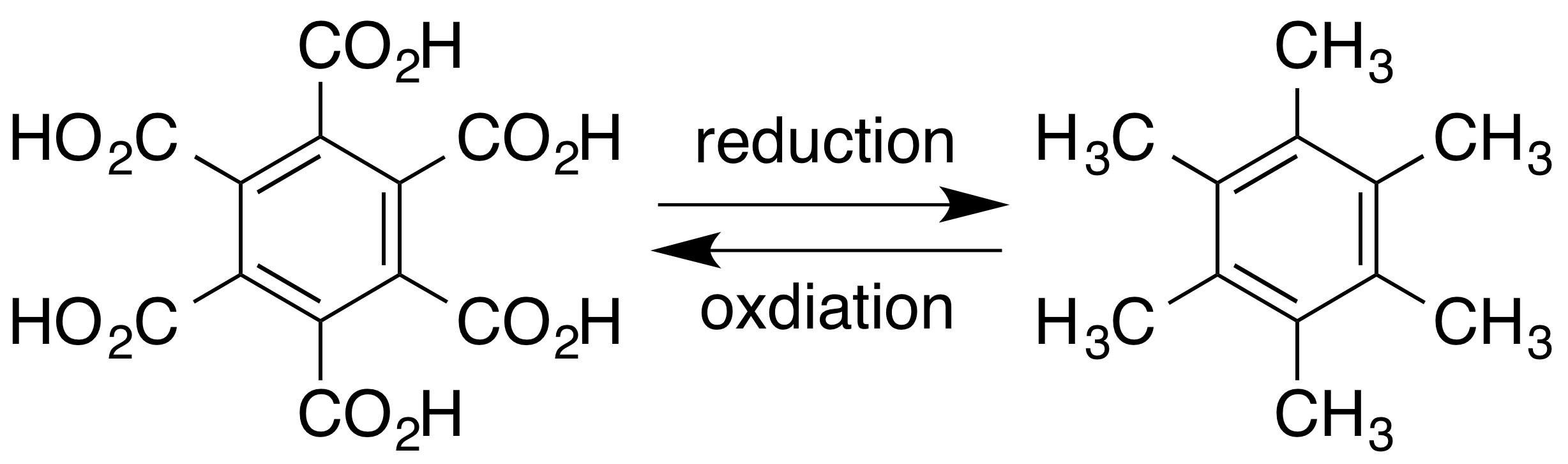
Hexamethylbenzene
Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929, Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity. Hexamethylbenzene can be oxidised to mellitic acid, which is found in nature as its aluminium salt in the rare mineral mellite. Hexamethylbenzene can be used as a ligand in organometallic compounds. An example from organoruthenium chemistry shows structural change in the ligand associated with changes in the oxidation state of the metal centre, though the same ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triptane
Triptane, or 2,2,3-trimethylbutane, is an organic chemical compound with the molecular formula C7 H16 or (H3C-)3C-C(-CH3)2H. It is therefore an alkane, specifically the most compact and heavily branched of the heptane isomers, the only one with a butane (C4) backbone. It was first synthesized in 1922 by Belgian chemists Georges Chavanne (1875–1941) and B. Lejeune, who called it trimethylisopropylmethane. Due to its high octane rating (112–113 RON, 101 MON) triptane was produced on alkylation units starting from 1943 for use as an anti-knock additive in gasoline. It was extensively researched for this role and received the modern name in the late 1930s at a joint laboratory of NACA, National Bureau of Standards, US Army Air Corps and the Bureau of Aeronautics. As of 2011, it was not a significant component of US automobile gasoline, present only in trace amounts (0.05–0.1%). See also *List of gasoline additives Gasoline additives may increase gasoline's octane rating ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, Volatility (chemistry), volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a Precursor (chemistry), precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl ''tert''-butyl ether, methyl benzoate, anisole, peroxyacids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmium Tetroxide
Osmium tetroxide (also osmium(VIII) oxide) is the chemical compound with the formula OsO4. The compound is noteworthy for its many uses, despite its toxicity and the rarity of osmium. It also has a number of unusual properties, one being that the solid is volatile. The compound is colourless, but most samples appear yellow. This is most likely due to the presence of the impurity osmium dioxide (OsO2), which is yellow-brown in colour. In biology, its property of binding to lipids has made it a widely used stain in electron microscopy. Physical properties Osmium(VIII) oxide forms monoclinic crystals. It has a characteristic acrid chlorine-like odor. The element name osmium is derived from ''osme'', Greek for ''odor''. OsO4 is volatile: it sublimes at room temperature. It is soluble in a wide range of organic solvents. It is moderately soluble in water, with which it reacts reversibly to form osmic acid (see below). ''Pure'' osmium(VIII) oxide is probably colourless; it has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a variety of materials (chemicals) depending on the type of cell. An electrode may be called either a cathode or anode according to the direction of the electric current, unrelated to the potential difference between electrodes. Michael Faraday coined the term "" in 1833; the word recalls the Greek ἤλεκτρον (, "amber") and ὁδός (, "path, way"). The electrophore, invented by Johan Wilcke in 1762, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Bromide
Zinc bromide ( Zn Br2) is an inorganic compound with the chemical formula Zn Br2. It is a colourless salt that shares many properties with zinc chloride (ZnCl2), namely a high solubility in water forming acidic solutions, and good solubility in organic solvents. It is hygroscopic and forms a dihydrate ZnBr2·2H2O. Production ZnBr2 · 2H2O is prepared by treating zinc oxide or zinc metal with hydrobromic acid. : ZnO + 2HBr + H2O → ZnBr2·2H2O : Zn + 2HBr → ZnBr2 + H2 The anhydrous material can be produced by dehydration of the dihydrate with hot CO2 or by reaction of zinc metal and bromine. Sublimation in a stream of hydrogen bromide also gives the anhydrous derivative. Structure ZnBr2 crystallizes in the same structure as ZnI2: four tetrahedral Zn centers share three vertices to form “super-tetrahedra” of nominal composition 2−, which are linked by their vertices to form a three-dimensional structure. The dihydrate ZnBr2 · 2H2O can be described as ( n(H2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Cell
An electrochemical cell is a device that either generates electrical energy from chemical reactions in a so called galvanic cell, galvanic or voltaic cell, or induces chemical reactions (electrolysis) by applying external electrical energy in an electrolytic cell. Both galvanic and electrolytic cells can be thought of as having two half-cells: consisting of separate Redox, oxidation and reduction reactions. When one or more electrochemical cells are connected in parallel or series they make a Battery (electricity), battery. Primary battery consists of single-use galvanic cells. Rechargeable batteries are built from #Secondary cells, secondary cells that use reversible reactions and can operate as galvanic cells (while providing energy) or electrolytic cells (while charging). Types of electrochemical cells Galvanic cell A galvanic cell (voltaic cell), named after Luigi Galvani (Alessandro Volta), is an electrochemical cell that generates electrical energy from spontaneous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patent
A patent is a type of intellectual property that gives its owner the legal right to exclude others from making, using, or selling an invention for a limited period of time in exchange for publishing an sufficiency of disclosure, enabling disclosure of the invention."A patent is not the grant of a right to make or use or sell. It does not, directly or indirectly, imply any such right. It grants only the right to exclude others. The supposition that a right to make is created by the patent grant is obviously inconsistent with the established distinctions between generic and specific patents, and with the well-known fact that a very considerable portion of the patents granted are in a field covered by a former relatively generic or basic patent, are tributary to such earlier patent, and cannot be practiced unless by license thereunder." – ''Herman v. Youngstown Car Mfg. Co.'', 191 F. 579, 584–85, 112 CCA 185 (6th Cir. 1911) In most countries, patent rights fall under private la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Opacity (optics)
Opacity is the measure of impenetrability to electromagnetic or other kinds of radiation, especially visible light. In radiative transfer, it describes the absorption and scattering of radiation in a medium, such as a plasma, dielectric, shielding material, glass, etc. An opaque object is neither transparent (allowing all light to pass through) nor translucent (allowing some light to pass through). When light strikes an interface between two substances, in general, some may be reflected, some absorbed, some scattered, and the rest transmitted (also see refraction). Reflection can be diffuse, for example light reflecting off a white wall, or specular, for example light reflecting off a mirror. An opaque substance transmits no light, and therefore reflects, scatters, or absorbs all of it. Other categories of visual appearance, related to the perception of regular or diffuse reflection and transmission of light, have been organized under the concept of cesia in an order sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |