|
Zinc Arsenide
Zinc arsenide (Zn3As2) is a binary compound of zinc with arsenic which forms gray tetragonal crystals. It is an inorganic semiconductor with a band gap of 1.0 eV. Synthesis and reactions Zinc arsenide can be prepared by the reaction of zinc with arsenic :3 Zn + 2 As → Zn3As2 Structure Zn3As2 has a room-temperature tetragonal form that converts to a different tetragonal phase at 190 °C and to a third phase at 651 °C. In the room-temperature form, the zinc atoms are tetrahedrally coordinated and the arsenic atoms are surrounded by six zinc atoms at the vertices of a distorted cube. The crystalline structure of zinc arsenide is very similar to that of cadmium arsenide (Cd3As2), zinc phosphide (Zn3P2) and cadmium phosphide (Cd3P2). These compounds of the Zn-Cd-P-As quaternary system exhibit full continuous solid-solution. Electronic structure Its lowest direct and indirect bandgaps are within 30 meV of each other. References arsenide zinc Zinc i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the Cube (geometry), cube becomes a rectangular Prism (geometry), prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The body-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
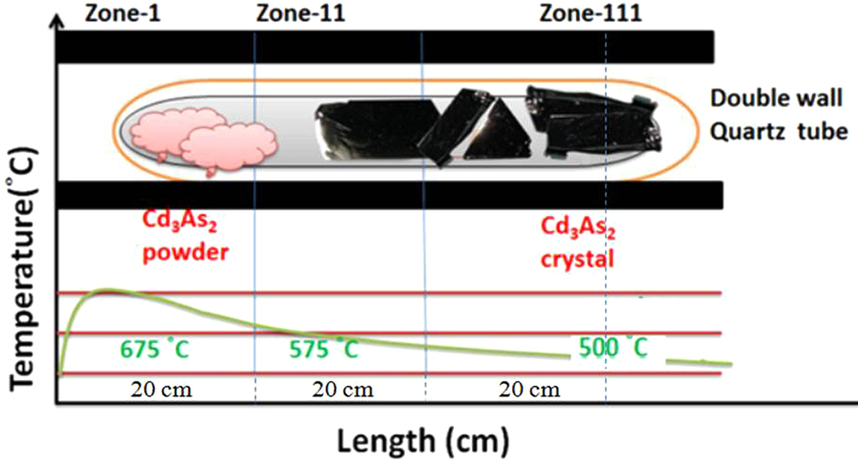
Cadmium Arsenide
Cadmium arsenide ( Cd3 As2) is an inorganic semimetal in the II-V family. It exhibits the Nernst effect. Properties Thermal Cd3As2 dissociates between 220 and 280 °C according to the reaction :2 Cd3As2(s) → 6 Cd(g) + As4(g) An energy barrier was found for the nonstoichiometric vaporization of arsenic due to the irregularity of the partial pressures with temperature. The range of the energy gap is from 0.5 to 0.6 eV. Cd3As2 melts at 716 °C and changes phase at 615 °C/ Phase transition Pure cadmium arsenide undergoes several phase transitions at high temperatures, making phases labeled α (stable), α’, α” (metastable), and β. At 593° the polymorphic transition α → β occurs. :α-Cd3As2 ↔ α’-Cd3As2 occurs at ~500 K. :α’-Cd3As2 ↔ α’’-Cd3As2 occurs at ~742 K and is a regular first order phase transition with marked hysteresis loop. :α”-Cd3As2 ↔ β-Cd3As2 occurs at 868 K. Single crystal x-ray diffraction was used to det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Compounds
Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 element, group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript appearance and behavior: they are generally colorless (unlike compounds of other elements with oxidation number +2, which are colored), do not readily engage in redox reactions, and generally adopt symmetrical structures. General characteristics In its compounds, Zn2+ ions have an electronic configuration [Ar] 3d10. As such, Zn2+ tends to have a symmetrical coordination geometry in both its complexes and compounds. In both ZnO and ZnS, (zincblende) zinc is bound tetrahedrally bound to four ligands (oxide and sulfide, respectively). Many coordination complex, complexes, such as ZnCl42−, are tetrahedral. Tetrahedrally coordinated zin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Cadmium Phosphide Arsenide
Zinc cadmium phosphide arsenide ( Zn- Cd- P- As) is a quaternary system of group II (IUPAC group 12) and group V (IUPAC group 15) elements. Many of the inorganic compounds in the system are II-V semiconductor materials. The quaternary system of II3V2 compounds, (Zn1−xCdx)3(P1−yAsy)2, has been shown to allow solid solution continuously over the whole compositional range. This material system and its subsets have applications in electronics, optoelectronics, including photovoltaics, and thermoelectrics. List of all binary compounds This system of elements contains numerous binary compounds and their solid solutions. Stable at atmospheric pressure The binary compounds thermodynamically stable at atmospheric pressure are listed in the following table: Metastable or unstable at atmospheric pressure Compounds metastable or unstable at atmospheric pressure are the following: Quaternary compounds The compounds of the form II3V2 have similar crystalline structures and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Phosphide
Cadmium phosphide ( Cd3 P2) is an inorganic chemical compound. It is a grey or white bluish solid semiconductor material with a bandgap of 0.5 eV. It has applications as a pesticide, material for laser diodes and for high-power-high-frequency electronics. Synthesis and reactions Cadmium phosphide can be prepared by the reaction of cadmium with phosphorus: :6 Cd + P4 → 2 Cd3P2 Structure Cd3P2 has a room-temperature tetragonal form. The crystalline structure of cadmium phosphide is very similar to that of zinc phosphide (Zn3P2), cadmium arsenide (Cd3As2) and zinc arsenide (Zn3As2). These compounds of the Zn-Cd-P-As quaternary system exhibit full continuous solid-solution. Applications Over the last decade, interest in cadmium phosphide as a source for fast, near-IR emission has grown due to the development of cadmium phosphide quantum dots. Literature has demonstrated that these quantum dots possess tunable emission between 700 nm to 1500 nm. A recent paper investi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Phosphide
Zinc phosphide ( Zn3 P2) is an inorganic chemical compound. It is a grey solid, although commercial samples are often dark or even black. It is used as a rodenticide. Zn3P2 is a II-V semiconductor with a direct band gap of 1.5 eV and may have applications in photovoltaic cells. A second compound exists in the zinc-phosphorus system, zinc diphosphide (ZnP2). Synthesis and reactions Zinc phosphide can be prepared by the reaction of zinc with phosphorus; however, for critical applications, additional processing to remove arsenic compounds may be needed. :6 Zn + P4 → 2 Zn3P2 Another method of preparation include reacting tri-n-octylphosphine with dimethylzinc. Zinc phosphide reacts with water to produce highly toxic phosphine (PH3) and zinc hydroxide (Zn(OH)2): :Zn3P2 + 6 H2O → 2 PH3 + 3 Zn(OH)2 Structure Zn3P2 has a room-temperature tetragonal form that converts to a cubic form at around 845 °C.Evgeniĭ I︠U︡rʹevich Tonkov, 1992, High Pressure Pha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal Crystal System
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The body-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binary Compound
In materials chemistry, a binary phase or binary compound is a chemical compound containing two different elements. Some binary phase compounds are molecular, e.g. carbon tetrachloride (CCl4). More typically binary phase refers to extended solids. Famous examples zinc sulfide Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various i ..., which contains zinc and sulfur, and tungsten carbide, which contains tungsten and carbon. Phases with higher degrees of complexity feature more elements, e.g. three elements in ternary phases, four elements in quaternary phases. These phases exhibit a higher degree of complexity due to the interaction of these elements at different conditions. References Chemical compounds {{chem-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Gap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to the energy difference (often expressed in electronvolts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote an electron from the valence band to the conduction band. The resulting conduction-band electron (and the electron hole in the valence band) are free to move within the crystal lattice and serve as charge carriers to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping levels are present in the same crystal, they form a semiconductor junction. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called " metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common semiconductor and is used in laser diodes, solar cells, microwave-frequency integrated circuits, and others. Silicon is a critical element for fabricating most electronic circuits. Semiconductor devices can display a range of different useful properties, such as passing current more easil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |