|
Zeisel Determination
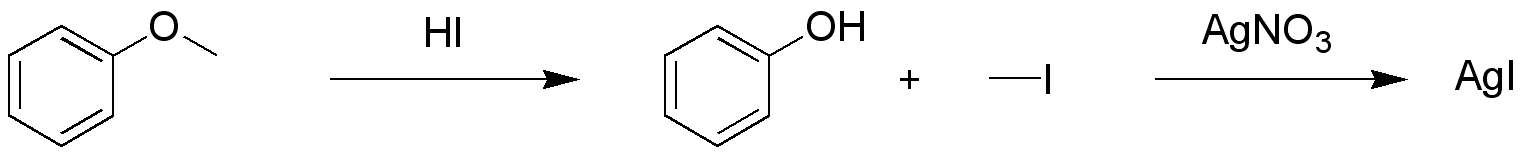
The Zeisel determination or Zeisel test is a chemical test for the presence of esters or ethers in a chemical substance A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent Chemical element, elements by physical separation m ....Lange (1962) ''J. Org. Chem.'' 27: 2037. It is named after the Czech chemist Simon Zeisel (1854–1933). In a qualitative test a sample is first reacted with a mixture of acetic acid and hydrogen iodide in a test tube. The ensuing reaction results in the cleavage of the ether or the ester into an alkyl iodide and respectively an Alcohol (chemistry), alcohol or a carboxylic acid. By heating this mixture, the gases are allowed to come into contact with a piece of paper higher up the test tube saturated with silver nitrate. Any alkyl iodide present will give a reaction with the silver compound to silver iodide which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Test
In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group. Purposes Chemical testing might have a variety of purposes, such as to: * Determine if, or verify that, the requirements of a specification, regulation, or contract are met * Decide if a new product development program is on track: Demonstrate proof of concept * Demonstrate the utility of a proposed patent * Determine the interactions of a sample with other known substances * Determine the composition of a sample * Provide standard data for other scientific, medical, and Quality assurance functions * Validate suitability for end-use * Provide a basis for Technical communication * Provide a technical means of comparison of several options * Provide evidence in legal proceedings Biochemical tests * Clinistrips quantitatively test for sugar in urine * The Kastle-Meyer test tests for the presence of blood * Salicyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Nitrate
Silver nitrate is an inorganic compound with chemical formula . It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called ''lunar caustic'' because silver was called ''luna'' by ancient alchemists who associated silver with the moon. In solid silver nitrate, the silver ions are three- coordinated in a trigonal planar arrangement. Synthesis and structure Albertus Magnus, in the 13th century, documented the ability of nitric acid to separate gold and silver by dissolving the silver. Indeed silver nitrate can be prepared by dissolving silver in nitric acid followed by evaporation of the solution. The stoichiometry of the reaction depends upon the concentration of nitric acid used. :3 Ag + 4 HNO3 (cold and diluted) → 3 AgNO3 + 2 H2O + NO :Ag + 2 HNO3 (hot and concentrated) → AgNO3 + H2O + NO2 The structure of silver nitrate has been examined by X-ray crystallography sev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(II) Iodide
Mercury(II) iodide is a chemical compound with the molecular formula Hg I2. It is typically produced synthetically but can also be found in nature as the extremely rare mineral coccinite. Unlike the related mercury(II) chloride it is hardly soluble in water (<100 ppm). Production Mercury(II) iodide is produced by adding an aqueous solution of to an aqueous solution of mercury(II) chloride with stirring; the precipitate is filtered off, washed and dried at 70 °C. : HgCl2 + 2 KI → HgI2 + 2 KClProperties Mercury(II) iodide displays |
Mercury(II) Nitrate
Mercury(II) nitrate is an inorganic compound with the formula Hg(NO3)2.xH2O. These colorless or white soluble crystalline salts are occasionally used as a reagent. It is made by treating mercury with hot concentrated nitric acid. Neither anhydrous nor monohydrate has been confirmed by X-ray crystallography. The anhydrous material is more widely used. Uses Mercuric nitrate has been used in mercuration of ketones. Mercuric nitrate was formerly used in carroting felt for hats. Health information Mercury compounds are highly toxic. The use of this compound by hatters and the subsequent mercury poisoning of said hatters is a common theory of where the phrase "mad as a hatter" came from. See also * The Hatter * Mercury poisoning * Gilding References External links ATSDR - Toxic Substances Portal - Mercury(11/14/2013) ATSDR - Public Health Statement: Mercury(11/14/2013) (link not traceable 11/14/2013) ATSDR - Medical Management Guidelines for Mercury(11/14/2013) ATSDR - Tox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methoxy
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula . On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position as an electron-donating group, but as an electron-withdrawing group if at the ''meta'' position. At the ''ortho'' position, steric effects are likely to cause a significant alteration in the Hammett equation prediction which otherwise follows the same trend as that of the ''para'' position. Occurrence The simplest of methoxy compounds are methanol and dimethyl ether. Other methoxy ethers include anisole and vanillin. Many alkoxides contain methoxy groups, e.g. tetramethyl orthosilicate and titanium methoxide. Such compounds are often classified as methoxides. Esters with a methoxy group can be referred to as methyl esters, and the —COOCH3 substituent is called a methoxycarbonyl. Biosynthesis In nature, methoxy groups are found on n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionized, for example by bombarding it with a beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragments) are then separated acco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Magnetic Resonance Spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds. The principle of NMR usually involves three sequential steps: # The alignment (polarization) of the magnetic nuclear spins in an applied, constant magnetic field B0. # The p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Iodide
Silver iodide is an inorganic compound with the formula Ag I. The compound is a bright yellow solid, but samples almost always contain impurities of metallic silver that give a gray coloration. The silver contamination arises because AgI is highly photosensitive. This property is exploited in silver-based photography. Silver iodide is also used as an antiseptic and in cloud seeding. Structure The structure adopted by silver iodide is temperature dependent: *Below 420 K, the β phase of AgI, with the wurtzite structure, is most stable. This phase is encountered in nature as the mineral iodargyrite. *Above 420 K, the α phase becomes more stable. This motif is a body-centered cubic structure which has the silver centers distributed randomly between 6 octahedral, 12 tetrahedral and 24 trigonal sites. At this temperature, Ag+ ions can move rapidly through the solid, allowing fast ion conduction. The transition between the β and α forms represents the melting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeisel01
Zeisel is a surname. Notable people with the surname include: *Eva Zeisel (1906–2011), Hungarian-born American industrial designer *Hans Zeisel Hans Zeisel (September 1, 1905 – March 7, 1992) was an Austrian-American sociologist and legal scholar who taught at the University of Chicago Law School from 1953 to 1974. He was best known for using quantitative social science techniques ... (1905–1992), American sociologist and legal scholar * Steven Zeisel, medical academic * Yeruham Zeisel (1909–1987), Israeli politician {{surname ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)
