Zeisel Determination on:
[Wikipedia]
[Google]
[Amazon]
The Zeisel determination or Zeisel test is a 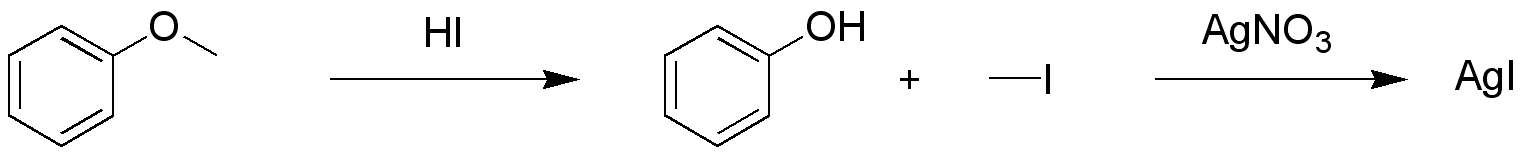 By heating this mixture, the gases are allowed to come into contact with a piece of paper higher up the test tube saturated with
By heating this mixture, the gases are allowed to come into contact with a piece of paper higher up the test tube saturated with 
Experimental procedure with anisole and methylbenzoate
chemical test
In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.
Purposes
Chemical testing might have a variety of purposes, such as to:
* Determine ...
for the presence of esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are ...
or ethers
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
in a chemical substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent Chemical element, elements by physical separation m ...
.Lange (1962) ''J. Org. Chem.'' 27: 2037. It is named after the Czech chemist Simon Zeisel (1854–1933). In a qualitative test a sample is first reacted with a mixture of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
and hydrogen iodide
Hydrogen iodide () is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under s ...
in a test tube
A test tube, also known as a culture tube or sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top and closed at the bottom.
Test tubes are usually placed in s ...
. The ensuing reaction results in the cleavage of the ether or the ester into an alkyl iodide
Organoiodine compounds are organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for he ...
and respectively an alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
or a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
.
 By heating this mixture, the gases are allowed to come into contact with a piece of paper higher up the test tube saturated with
By heating this mixture, the gases are allowed to come into contact with a piece of paper higher up the test tube saturated with silver nitrate
Silver nitrate is an inorganic compound with chemical formula . It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called ''lunar caustic ...
. Any alkyl iodide present will give a reaction with the silver compound to silver iodide
Silver iodide is an inorganic compound with the formula Ag I. The compound is a bright yellow solid, but samples almost always contain impurities of metallic silver that give a gray coloration. The silver contamination arises because AgI is hig ...
which has a red or yellow color. By filtering and weighing this precipitate it is possible to quantitatively calculate the number of iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
atoms and hence alkoxy groups. For example, prior to the development of the more precise methods of NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fiel ...
and mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
, the Zeisel test was widely used to determine the number of methoxy
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula .
On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position ...
(-OCH3) and ethoxy (-OCH2CH3) groups in carbohydrate and organophosphorus
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective in ...
insecticides.
An alternative qualitative Zeisel test can be done with the use of mercury(II) nitrate
Mercury(II) nitrate is an inorganic compound with the formula Hg(NO3)2.xH2O. These colorless or white soluble crystalline salts are occasionally used as a reagent. It is made by treating mercury with hot concentrated nitric acid. Neither anhyd ...
instead of silver nitrate, leading to the formation of scarlet red mercury(II) iodide
Mercury(II) iodide is a chemical compound with the molecular formula Hg I2. It is typically produced synthetically but can also be found in nature as the extremely rare mineral coccinite. Unlike the related mercury(II) chloride it is hardly sol ...
.
Synthetic applications:

External links
Experimental procedure with anisole and methylbenzoate
References
{{Reflist Chemical tests