|
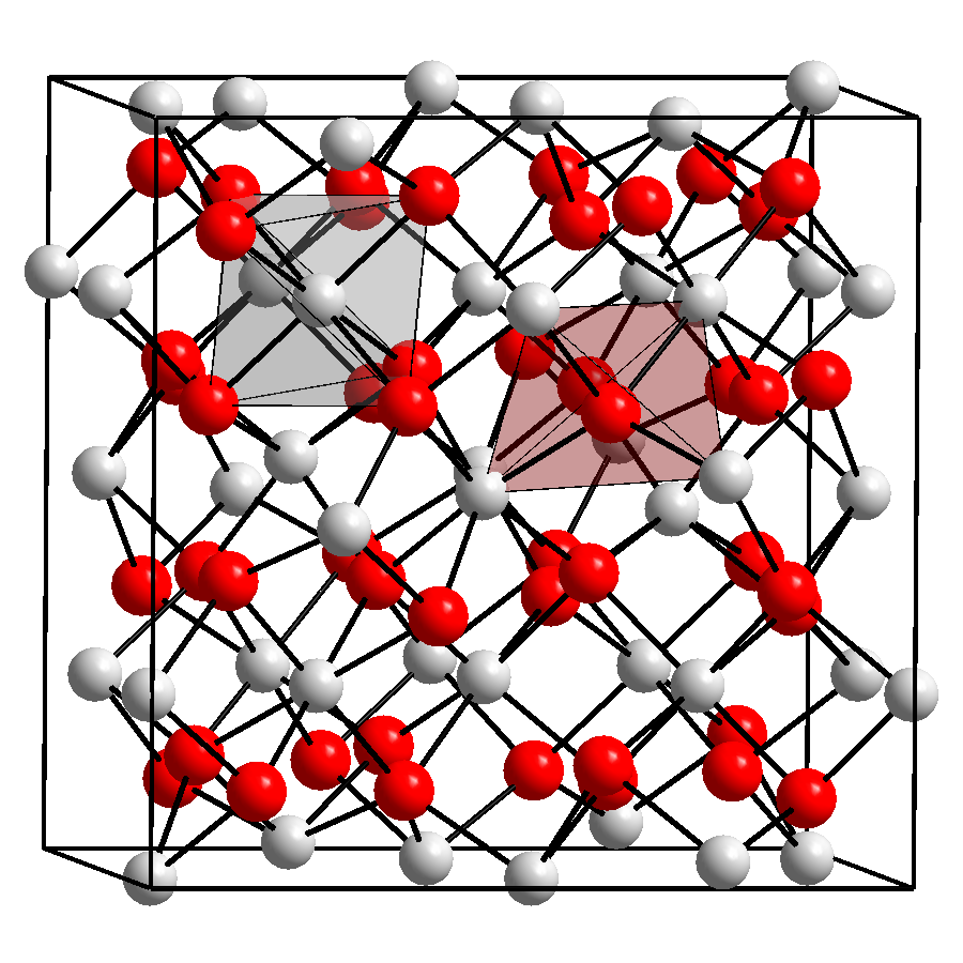
Ytterbium Tribromide
Ytterbium(III) bromide ( Yb Br3) is an inorganic chemical compound. Refer to the adjacent table for the main properties of Ytterbium(III) bromide. Preparation Dissolving ytterbium oxide into 40% hydrobromic acid forms YbBr3·6H2O crystals. After mixing the hydrate with ammonium bromide Ammonium bromide, NH4Br, is the ammonium salt of hydrobromic acid. The chemical crystallizes in colorless prisms, possessing a saline taste; it sublimes on heating and is easily soluble in water. On exposure to air it gradually assumes a yellow ... and heating it in a vacuum, anhydrous YbBr3 can be obtained.林平娣, 吴国庆无水三溴化钐和三溴化镱的制备 化学试剂, 1991(1):13-14. : Yb2O3 + 6 HBr → 2 YbBr3 + 3 H2O Ytterbium(III) bromide can also be prepared by directly heating ytterbium oxide and ammonium bromide. References Bromides Lanthanide halides Ytterbium(III) compounds {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trigonal
In crystallography, the hexagonal crystal family is one of the six crystal family, crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section hexagonal crystal family#Crystal systems, crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure. It was originated by William Burton Pearson and is used extensively in Pearson's handbook of crystallographic data for intermetallic phases. The symbol is made up of two letters followed by a number. For example: * Diamond structure, cF8 * Rutile structure, tP6 Construction The two letters in the Pearson symbol specify the Bravais lattice, and more specifically, the lower-case letter specifies the Crystal system, crystal family, while the upper-case letter the Lattice (group), lattice type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell (atoms which satisfy 1 > x,y,z \geq 0 for the atom's position (x,y,z) in the unit cell). [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ytterbium
Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density, melting point and boiling point are much lower than those of most other lanthanides. In 1878, Swiss chemist Jean Charles Galissard de Marignac separated from the rare earth "erbia", another independent component, which he called " ytterbia", for Ytterby, the village in Sweden near where he found the new component of erbium. He suspected that ytterbia was a compound of a new element that he called "ytterbium". Four elements were named after the village, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived , referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a free element in nature. Instead, it can be isolated from colourless soluble crystalline mineral halide Ionic salt, salts analogous to table salt, a property it shares with the other halogens. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its Bromine cycle, accumulation in the oceans. Commercially the element is easily extracted from brine evaporation ponds, mostly in the United States and Israel. The mass of bromine in the oce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within living things. History Friedrich Wöhler's con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ytterbium Oxide
Ytterbium(III) oxide is the chemical compound with the formula Yb2O3. It is one of the more commonly encountered compounds of ytterbium. It occurs naturally in trace amounts in the mineral gadolinite. It was first isolated from this in 1878 by Jean Charles Galissard de Marignac. Preparation Ytterbium(III) oxide can be obtained by directly reacting ytterbium with oxygen: : It can also be obtained by the thermal decomposition of ytterbium carbonate or ytterbium oxalate at temperatures around 700 °C: : : Properties Chemical Ytterbium(III) oxide is a white powder. It reacts with carbon tetrachloride or hot hydrochloric acid to form ytterbium(III) chloride: : : Physical Like the other trivalent oxides of the heavier lanthanides, ytterbium(III) oxide has the "rare-earth C-type sesquioxide" structure which is related to the fluorite structure with one quarter of the anions removed, leading to ytterbium atoms in two different six coordinate (non-octahedral) env ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrobromic Acid
Hydrobromic acid is an aqueous solution of hydrogen bromide. It is a strong acid formed by dissolving the diatomic molecule hydrogen bromide (HBr) in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at and contains 47.6% HBr by mass, which is 8.77 mol/L. Hydrobromic acid is one of the strongest mineral acids known. Uses Hydrobromic acid is mainly used for the production of inorganic bromides, especially the bromides of zinc, calcium, and sodium. It is a useful reagent for generating organobromine compounds. Certain ethers are cleaved with HBr. It also catalyzes alkylation reactions and the extraction of certain ores. Industrially significant organic compounds prepared from hydrobromic acid include allyl bromide, tetrabromobis(phenol), and bromoacetic acid. HBr participates in anti-Markovnikov hydrohalogenation of alkenes in the presence of peroxides. The resulting 1-bromoalkanes are versatile alkylating agents, giving rise to fatty amines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Bromide
Ammonium bromide, NH4Br, is the ammonium salt of hydrobromic acid. The chemical crystallizes in colorless prisms, possessing a saline taste; it sublimes on heating and is easily soluble in water. On exposure to air it gradually assumes a yellow color because of the oxidation of bromide (Br−) to bromine (Br2). Preparation Ammonium bromide can be prepared by the direct action of hydrogen bromide on ammonia. : NH3 + HBr → NH4Br It can also be prepared by the reaction of ammonia with iron(II) bromide or iron(III) bromide, which may be obtained by passing aqueous bromine solution over iron filings. : 2 NH3 + FeBr2 + 2 H2O → 2 NH4Br + Fe(OH)2 Reactions Ammonium bromide is a weak acid with a p''K''a of approximately 9 in water. It is an acid salt because the ammonium ion hydrolyzes slightly in water. Ammonium bromide is a strong electrolyte when put in water: :NH4Br(s) → (aq) + Br−(aq) Ammonium bromide decomposes to ammonia and hydrogen bromide when heated at elev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromides
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant materials, and cell stains. Although uncommon, chronic toxicity from bromide can result in bromism, a syndrome with multiple neurological symptoms. Bromide toxicity can also cause a type of skin eruption, see potassium bromide. The bromide ion has an ionic radius of 196 pm. Natural occurrence Bromide is present in typical seawater (35 PSU) with a concentration of around 65 mg/L, which is about 0.2% of all dissolved salts. Seafood and deep sea plants generally have higher levels than land-derived foods. Bromargyrite—natural, crystalline silver bromide—is the most common bromide mineral known but is still very rare. In addition to silver, bromine is also in minerals combined with mercury and copper. Formation and re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide Halides
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (element 71) is also sometimes considered a lanthanide, despite being a d-block element and a transition metal. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. Lutetium is a d-block element (thus also a transition metal), and on this basis its inclusion has been questioned; however, like its congeners scandium and yttrium in group 3, it behaves similarly to the other 14. The term rare-earth element or rare-earth metal is often used to include the stable group 3 elements Sc, Y, and Lu in addition to the 4f elements. All lanthanide elements form trivalent cations, Ln3+, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |