|
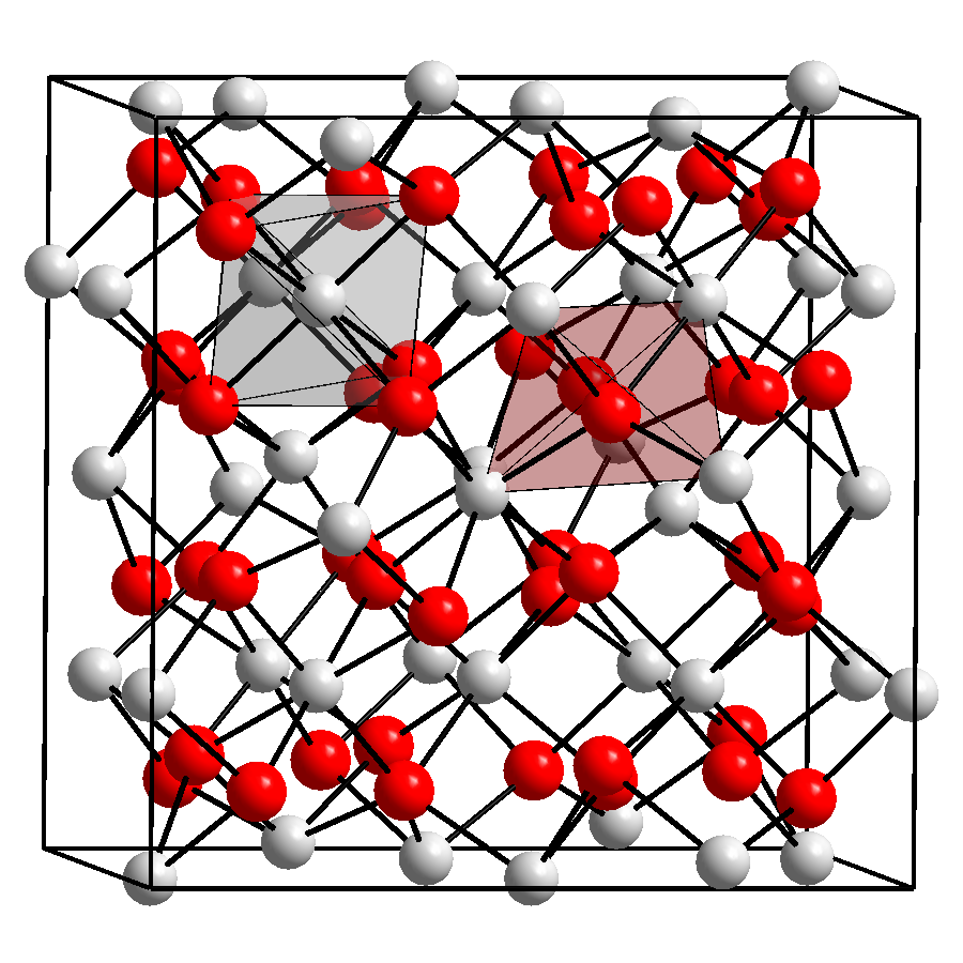
Ytterbium Oxide
Ytterbium(III) oxide is the chemical compound with the formula Yb2O3. It is one of the more commonly encountered compounds of ytterbium. It occurs naturally in trace amounts in the mineral gadolinite. It was first isolated from this in 1878 by Jean Charles Galissard de Marignac. Preparation Ytterbium(III) oxide can be obtained by directly reacting ytterbium with oxygen: : It can also be obtained by the thermal decomposition of ytterbium carbonate or ytterbium oxalate at temperatures around 700 °C: : : Properties Chemical Ytterbium(III) oxide is a white powder. It reacts with carbon tetrachloride or hot hydrochloric acid to form ytterbium(III) chloride: : : Physical Like the other trivalent oxides of the heavier lanthanides, ytterbium(III) oxide has the "rare-earth C-type sesquioxide" structure which is related to the fluorite structure with one quarter of the anions removed, leading to ytterbium atoms in two different six coordinate (non-octahedral) env ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ytterbium(III) Sulfide
Ytterbium(III) sulfide is a binary inorganic compound of ytterbium and sulfur with the chemical formula . Preparation Ytterbium(III) sulfide can be obtained by reacting ytterbium and sulfur in an inert atmosphere at 450–800 °C: :: It can also be prepared by passing hydrogen sulfide Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ... through heated ytterbium(III) oxide: :: Physical properties Ytterbium(III) sulfide forms yellow crystals of rhombic symmetry, and cell parameters a = 0.678 nm, b = 0.995 nm, c = 0.361 nm. Uses This salt is used in the production of ceramics and as a catalyst. References Ytterbium(III) compounds Sulfur compounds Sesquisulfides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass are named after the material, e.g., a Tumbler (glass), "glass" for drinking, "glasses" for vision correction, and a "magnifying glass". Glass is most often formed by rapid cooling (quenching) of the Melting, molten form. Some glasses such as volcanic glass are naturally occurring, and obsidian has been used to make arrowheads and knives since the Stone Age. Archaeological evidence suggests glassmaking dates back to at least 3600 BC in Mesopotamia, Ancient Egypt, Egypt, or Syria. The earliest known glass objects were beads, perhaps created accidentally during metalworking or the production of faience, which is a form of pottery using lead glazes. Due to its ease of formability int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ytterbium(III) Compounds
Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density, melting point and boiling point are much lower than those of most other lanthanides. In 1878, Swiss chemist Jean Charles Galissard de Marignac separated from the rare earth "erbia", another independent component, which he called "ytterbia", for Ytterby, the village in Sweden near where he found the new component of erbium. He suspected that ytterbia was a compound of a new element that he called "ytterbium". Four elements were named after the village, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Active Laser Medium
The active laser medium (also called a gain medium or lasing medium) is the source of optical gain within a laser. The gain results from the stimulated emission of photons through electronic or molecular transitions to a lower energy state from a higher energy state previously populated by a pump source. Examples of active laser media include: * Certain crystals, typically doped with rare-earth ions (e.g. neodymium, ytterbium, or erbium) or transition metal ions (titanium or chromium); most often yttrium aluminium garnet ( Y3 Al5 O12), yttrium orthovanadate (YVO4), or sapphire (Al2O3); and not often caesium cadmium bromide ( Cs Cd Br3) (solid-state lasers) * Glasses, e.g. silicate or phosphate glasses, doped with laser-active ions; * Gases, e.g. mixtures of helium and neon (HeNe), nitrogen, argon, krypton, carbon monoxide, carbon dioxide, or metal vapors; ( gas lasers) * Semiconductors, e.g. gallium arsenide (GaAs), indium gallium arsenide (InGaAs), or gallium nitride (GaN ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dielectric
In electromagnetism, a dielectric (or dielectric medium) is an Insulator (electricity), electrical insulator that can be Polarisability, polarised by an applied electric field. When a dielectric material is placed in an electric field, electric charges do not flow through the material as they do in an electrical conductor, because they have no loosely bound, or free, electrons that may drift through the material, but instead they shift, only slightly, from their average equilibrium positions, causing dielectric polarisation. Because of Polarisation density, dielectric polarisation, positive charges are displaced in the direction of the field and negative charges shift in the direction opposite to the field. This creates an internal electric field that reduces the overall field within the dielectric itself. If a dielectric is composed of weakly Chemical bond, bonded molecules, those molecules not only become polarised, but also reorient so that their Symmetry axis, symmetry axes a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optical Fiber
An optical fiber, or optical fibre, is a flexible glass or plastic fiber that can transmit light from one end to the other. Such fibers find wide usage in fiber-optic communications, where they permit transmission over longer distances and at higher Bandwidth (computing), bandwidths (data transfer rates) than electrical cables. Fibers are used instead of metal wires because signals travel along them with less Attenuation, loss and are immune to electromagnetic interference. Fibers are also used for illumination (lighting), illumination and imaging, and are often wrapped in bundles so they may be used to carry light into, or images out of confined spaces, as in the case of a fiberscope. Specially designed fibers are also used for a variety of other applications, such as fiber optic sensors and fiber lasers. Glass optical fibers are typically made by Drawing (manufacturing), drawing, while plastic fibers can be made either by drawing or by extrusion. Optical fibers typically incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radiation. The first laser was built in 1960 by Theodore Maiman at Hughes Research Laboratories, based on theoretical work by Charles H. Townes and Arthur Leonard Schawlow and the optical amplifier patented by Gordon Gould. A laser differs from other sources of light in that it emits light that is coherence (physics), ''coherent''. Spatial coherence allows a laser to be focused to a tight spot, enabling uses such as optical communication, laser cutting, and Photolithography#Light sources, lithography. It also allows a laser beam to stay narrow over great distances (collimated light, collimation), used in laser pointers, lidar, and free-space optical communication. Lasers can also have high temporal coherence, which permits them to emit light ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Garnet
Garnets () are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives. Garnet minerals, while sharing similar physical and crystallographic properties, exhibit a wide range of chemical compositions, defining distinct species. These species fall into two primary solid solution series: the pyralspite series (pyrope, almandine, spessartine), with the general formula [Mg,Fe,Mn]3Al2(SiO4)3; and the ugrandite series (uvarovite, grossular, andradite), with the general formula Ca3[Cr,Al,Fe]2(SiO4)3. Notable varieties of grossular include Grossular#Hessonite, hessonite and tsavorite. Etymology The word ''garnet'' comes from the 14th-century Middle English word ''gernet'', meaning 'dark red'. It is borrowed from Old French ''grenate'' from Latin language, Latin ''granatus,'' from ''granum'' ('grain, seed'). This is possibly a reference to ''mela granatum'' or even ''pomum granatum'' ('pomegranate', ''Punica granatum''), a plant whose fruits conta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopant
A dopant (also called a doping agent) is a small amount of a substance added to a material to alter its physical properties, such as electrical or optics, optical properties. The amount of dopant is typically very low compared to the material being doped. When doped into crystalline substances, the dopant's atoms get incorporated into the crystal lattice of the substance. The crystalline materials are frequently either crystals of a semiconductor such as silicon and germanium for use in solid-state electronics, or transparency and translucency, transparent crystals for use in the production of various laser types; however, in some cases of the latter, noncrystalline substances such as glass can also be doped with impurities. In solid-state electronics using the proper types and amounts of dopants in semiconductors is what produces the p-type semiconductors and n-type semiconductors that are essential for making transistors and diodes. Transparent crystals Lasing media The p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitreous Enamel
Vitreous enamel, also called porcelain enamel, is a material made by melting, fusing powdered glass to a substrate by firing, usually between . The powder melts, flows, and then hardens to a smooth, durable vitrification, vitreous coating. The word ''vitreous'' comes from the Latin , meaning "glassy". Enamel can be used on metal, enamelled glass, glass, overglaze decoration, ceramics, stone, or any material that will withstand the fusing temperature. In technical terms fired enamelware is an integrated layered composite of glass and another material (or more glass). The term "enamel" is most often restricted to work on metal, which is the subject of this article. Essentially the same technique used with other bases is known by different terms: on glass as ''enamelled glass'', or "painted glass", and on pottery it is called ''overglaze decoration'', "overglaze enamels" or "enamelling". The craft is called "enamelling", the artists "enamellers" and the objects produced can be cal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ytterbium(III) Chloride
Ytterbium(III) chloride ( Yb Cl3) is an inorganic compound. It was first synthesized by Jan Hoogschagen in 1946. It is a paramagnetic Lewis acid, like many of the lanthanide chlorides. This gives rise to pseudocontact shifted NMR spectra, akin to NMR shift reagents. It reacts with NiCl2 to form a very effective catalyst for the reductive dehalogenation of aryl halides. Chemical properties The valence electron configuration of Yb+3 (from YbCl3) is 4''f''135''s''25''p''6, which has crucial implications for the chemical behaviour of Yb+3. Also, the size of Yb+3 governs its catalytic behaviour and biological applications. For example, while both Ce+3 and Yb+3 have a single unpaired ''f'' electron, Ce+3 is much larger than Yb+3 because lanthanides become much smaller with increasing effective nuclear charge as a consequence of the ''f'' electrons not being as well shielded as ''d'' electrons. This behavior is known as the lanthanide contraction. The small size of Yb+3 produces fast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |