|
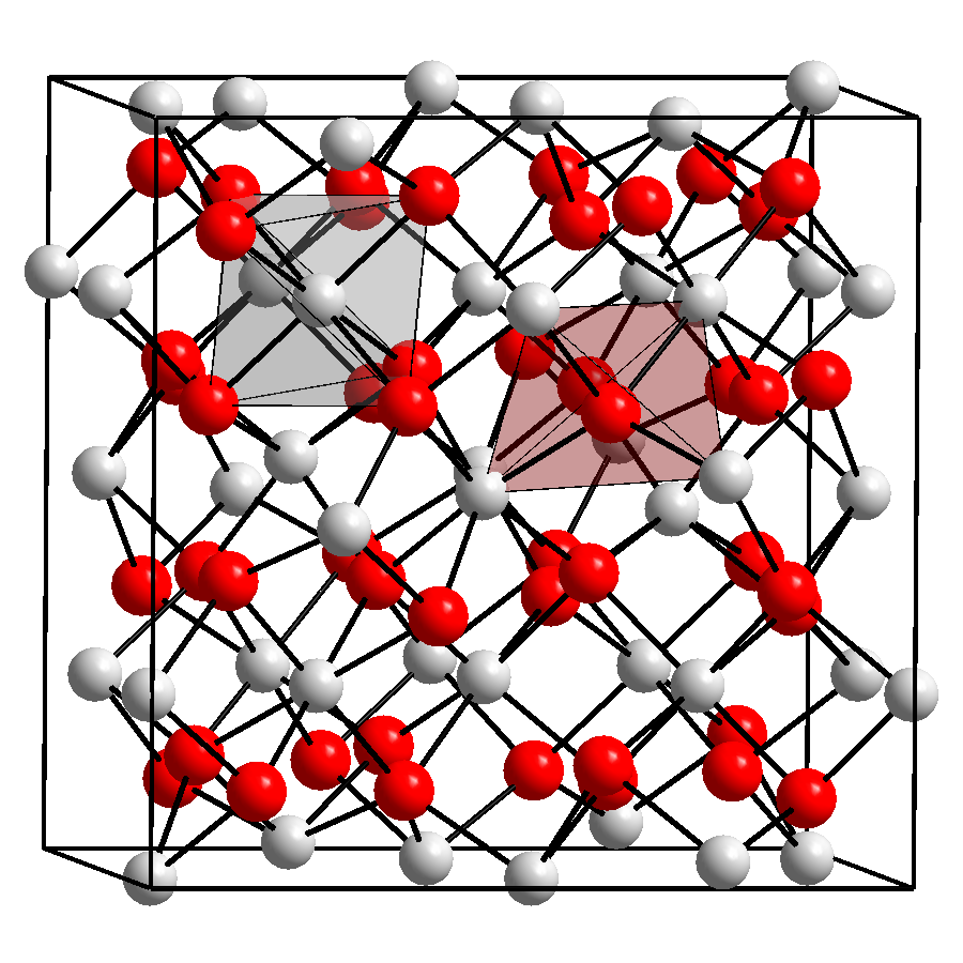
Ytterbium Nitride
Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density, melting point and boiling point are much lower than those of most other lanthanides. In 1878, Swiss chemist Jean Charles Galissard de Marignac separated from the rare earth "erbia", another independent component, which he called "ytterbia", for Ytterby, the village in Sweden near where he found the new component of erbium. He suspected that ytterbia was a compound of a new element that he called "ytterbium". Four elements were named after the village, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its atomic nucleus, nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements form Homonuclear molecule, molecules of atoms of said element only: e.g. atoms of hydrogen (H) form Diatomic molecule, diatomic molecules (H). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charles James (chemist)
Charles James (27 April 1880 – 10 December 1928) was a chemist of British origin working in the United States. He became a professor and head of the chemistry department at the New Hampshire College of Agriculture and the Mechanic Arts (now the University of New Hampshire) in Durham, New Hampshire, US. James developed the James method for the separation and identification of rare-earth elements by fractional precipitation and crystallization, and provided extracted elements to researchers worldwide. James was one of the first scientists to identify element 71, later named lutetium, and believed that he had found the final rare earth element 61, later named promethium. In 1999 the American Chemical Society recognized Charles James's work in chemical separations as a National Historic Chemical Landmark. Early life Charles James was born on 27 April 1880 to William James and Mary Diana Shatford-James in Earls Barton near Wellingborough, Northamptonshire . His father died whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropy
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical State of matter, state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the atoms of the element are Chemical bond, bonded together in different manners. For example, the allotropes of carbon include diamond (the carbon atoms are bonded together to form a Cubic crystal system, cubic lattice of Tetrahedral molecular geometry, tetrahedra), graphite (the carbon atoms are bonded together in sheets of a hexagonal lattice), graphene (single sheets of graphite), and fullerenes (the carbon atoms are bonded together in spherical, tubular, or ellipsoidal formations). The term ''allotropy'' is used for elements only, not for Chemical compound, compounds. The more general term, used for any compound, is Polymorphism (materials science), polymorphism, although its use is usually restricted to solid materials ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral Acid
A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds, as opposed to organic acids which are acidic, organic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water. Characteristics Commonly used mineral acids are sulfuric acid (H2SO4), hydrochloric acid (HCl) and nitric acid (HNO3); these are also known as bench acids. Mineral acids range from superacids (such as perchloric acid) to very weak ones (such as boric acid). Mineral acids tend to be very soluble in water and insoluble in organic solvents. Mineral acids are used in many sectors of the chemical industry as feedstocks for the synthesis of other chemicals, both organic and inorganic. Large quantities of these acids—especially sulfuric acid, nitric acid, and hydrochloric acid—are manufactured for commercial use in large plants. Mineral acids are also used directly for their corrosive properties. For example, a dilute solution of hydrochl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare-earth Element
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set of 17 nearly indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. The term "rare-earth" is a misnomer because they are not actually scarce, but historically it took a long time to isolate these elements. They are relatively plentiful in the entire Earth's crust (cerium being the 25th-most-abundant element at 68 parts per million, more abundant than copper), but in practice they are spread thinly as trace impurities, so to obtain rare earths at usable purity requires processing enormous amounts of raw ore at great expense; thus the name "rare" earths. Scandium and yttrium are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ductility
Ductility refers to the ability of a material to sustain significant plastic Deformation (engineering), deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic deformation, which is reversible upon removing the stress. Ductility is a critical mechanical performance indicator, particularly in applications that require materials to bend, stretch, or deform in other ways without breaking. The extent of ductility can be quantitatively assessed using the percent elongation at break, given by the equation: \% \mathrm= \left ( \frac \right )\times100 where l_ is the length of the material after fracture and l_0 is the original length before testing. This formula helps in quantifying how much a material can stretch under tensile stress before failure, providing key insights into its ductile behavior. Ductility is an important consideration in engineering and manufacturing. It defines a material's suitabil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare-earth Elements
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set of 17 nearly indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. The term "rare-earth" is a misnomer because they are not actually scarce, but historically it took a long time to isolate these elements. They are relatively plentiful in the entire Earth's crust (cerium being the 25th-most-abundant element at 68 parts per million, more abundant than copper), but in practice they are spread thinly as trace impurities, so to obtain rare earths at usable purity requires processing enormous amounts of raw ore at great expense; thus the name "rare" earths. Scandium and yttrium are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenotime
Xenotime is a rare-earth phosphate mineral, the major component of which is yttrium orthophosphate ( Y P O4). The phosphate ions are described by a tetrahedral shape and coordinate to the center Y3+ metal ion in a way that closely resembles the structure of zircon (ZrSiO4). It forms a solid solution series with chernovite-(Y) ( Y As O4) and therefore may contain trace impurities of arsenic, as well as silicon dioxide and calcium. Other iso-structural ions that undergo exchanges with PO4 are VO4 and NbO4 ions, contributing to the list of possible co-occurring elements that may be in need of separation. The rare-earth elements dysprosium, erbium, terbium and ytterbium, as well as metal elements such as thorium and uranium (all replacing yttrium) are the expressive secondary components of xenotime. Due to uranium and thorium impurities, some xenotime specimens may be weakly to strongly radioactive. Lithiophyllite, monazite and purpurite are sometimes grouped with xenotime in the in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Euxenite
Euxenite, or euxenite-(Y) (the official mineralogical name), is a brownish black mineral with a metallic luster. Chemistry It contains calcium, niobium, tantalum, cerium, titanium, yttrium, and typically uranium and thorium, with some other metals. The chemical formula is . It is commonly partially amorphous due to radiation damage. Euxenite forms a continuous series with the titanium rich polycrase-(Y) having the formula . Name and discovery It was first described in 1870 and named for from the Greek (εὔξενος), ''hospitable'' or ''friendly to strangers'', in allusion to the many rare elements that it contains. Occurrence It occurs in granite pegmatites and detrital black sands. It is found in many locations worldwide, notably its type locality in Jølster, Sunnfjord, Norway. Other locations include the Ural Mountains of Russia; Sweden; Minas Gerais, Brazil; Ampangabe, Madagascar; Ontario, Canada; and in Arizona, Wyoming and Colorado in the US.http://www.galleries. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium-dominant member of the group. It occurs usually in small isolated crystals. It has a hardness of 5.0 to 5.5 on the Mohs scale of mineral hardness and is relatively dense, about 4.6 to 5.7 g/cm3. There are five different most common species of monazite, depending on the relative amounts of the rare earth elements in the mineral: * monazite-(Ce), (the most common member), * monazite-(La), , * monazite-(Nd), , * monazite-(Sm), , * monazite-(Pr), . The elements in parentheses are listed in the order of their relative proportion within the mineral: lanthanum is the most common rare-earth element in monazite-(La), and so forth. Silica () is present in trace amounts, as well as small amounts of uranium and thorium. Due to the alpha decay o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parts Per Million
In science and engineering, the parts-per notation is a set of pseudo-units to describe the small values of miscellaneous dimensionless quantity, dimensionless quantities, e.g. mole fraction or mass fraction (chemistry), mass fraction. Since these fraction (mathematics), fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement. Commonly used are * parts-per-million - ppm, * parts-per-billion - ppb, * parts-per-trillion - ppt, * parts-per-quadrillion - ppq, This notation is not part of the International System of Units - SI system and its meaning is ambiguous. Applications Parts-per notation is often used describing dilute solutions in chemistry, for instance, the relative abundance of dissolved minerals or pollutants in water. The quantity "1 ppm" can be used for a mass fraction if a water-borne pollutant is present at one-millionth of a gram per gram of sample solution. When working with aqueous solutions, it is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |