|
Ytterbium(II) Iodide
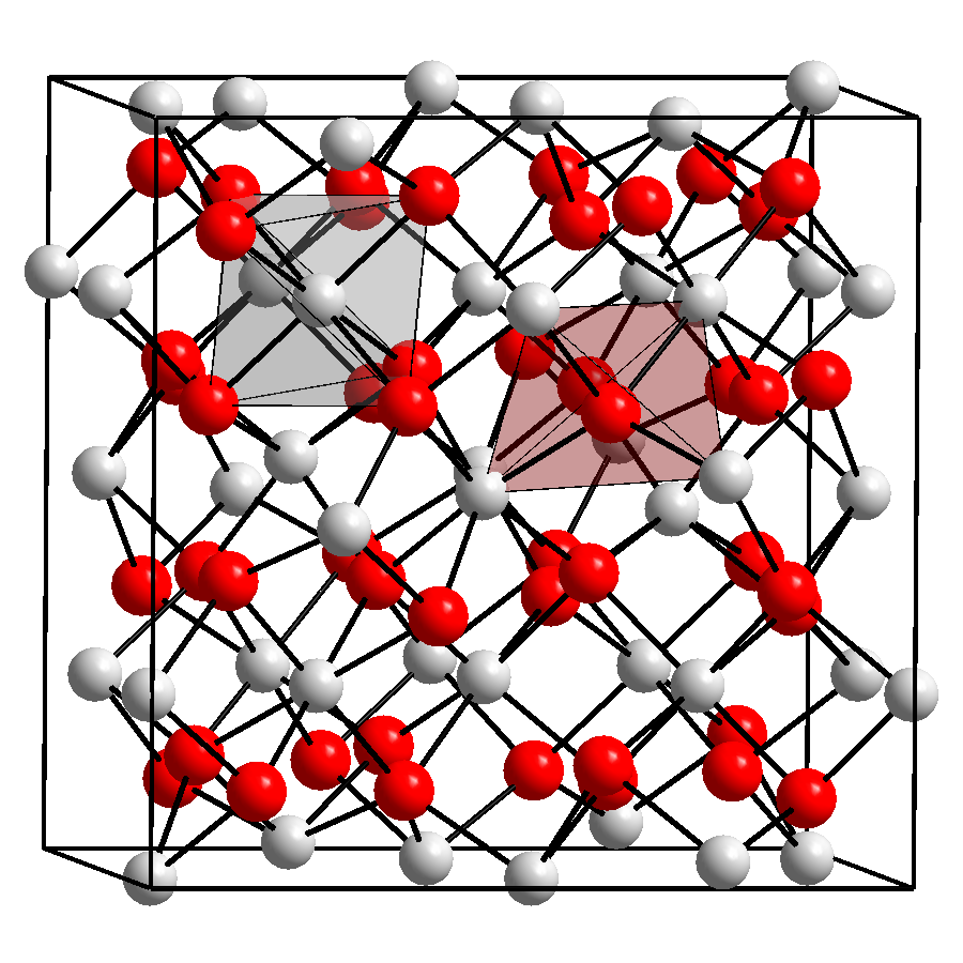
Ytterbium(II) iodide is an iodide of ytterbium, with the chemical formula of YbI2. It is a yellow solid. Preparation Ytterbium(II) iodide can be prepared by heating ytterbium(III) iodide: :\mathrm It can also be prepared by reacting metallic ytterbium with 1,2-diiodoethane in tetrahydrofuran: :\mathrm Although the reaction takes place at room temperature, due to the sensitivity of the reagents it is necessary to work anhydrous and under inert gas. Otherwise, if oxygen is present, rapid oxidation to ytterbium(III) takes place. This can be visually recognized by the color change from green to yellow solution. Properties and uses Ytterbium(II) iodide is a yellow solid that is very sensitive to air and moisture and is rapidly oxidized to ytterbium(III). It reacts with water to produce hydrogen gas and basic iodides, and reacts violently with acids. Ytterbium(II) iodide sinters at 0.01 Torr from about 780 °C and gives a viscous melt at about 920 °C. It begin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trigonal
In crystallography, the hexagonal crystal family is one of the six crystal family, crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section hexagonal crystal family#Crystal systems, crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is not. The l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ytterbium
Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density, melting point and boiling point are much lower than those of most other lanthanides. In 1878, Swiss chemist Jean Charles Galissard de Marignac separated from the rare earth "erbia", another independent component, which he called " ytterbia", for Ytterby, the village in Sweden near where he found the new component of erbium. He suspected that ytterbia was a compound of a new element that he called "ytterbium". Four elements were named after the village, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ytterbium(III) Iodide
Ytterbium(III) iodide is one of ytterbium's iodides, with the chemical formula of YbI3. Preparation Ytterbium(III) iodide can be prepared by reacting metallic ytterbium with iodine at 500°C with a 30 atm pressure: : 2 Yb + 3 I2 → 2 YbI3 Ytterbium(III) oxide, ytterbium(III) hydroxide or ytterbium(III) carbonate can react with hydroiodic acid to obtain ytterbium(III) iodide in aqueous solution: : Yb2O3 + 6 HI → 2 YbI3 + 3 H2O : Yb(OH)3 + 3 HI → YbI3 + 3 H2O : Yb2(CO3)3 + 6 HI → 2 YbI3 + 3 H2O + 3 CO2 The ytterbium(III) iodide hydrate crystallized from the solution can be heated with ammonium iodide to obtain the anhydrous form. Reactions Ytterbium(III) iodide decomposes to ytterbium(II) iodide Ytterbium(II) iodide is an iodide of ytterbium, with the chemical formula of YbI2. It is a yellow solid. Preparation Ytterbium(II) iodide can be prepared by heating ytterbium(III) iodide: :\mathrm It can also be prepared by reacting metalli ... upon heating: :2 YbI ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-diiodoethane
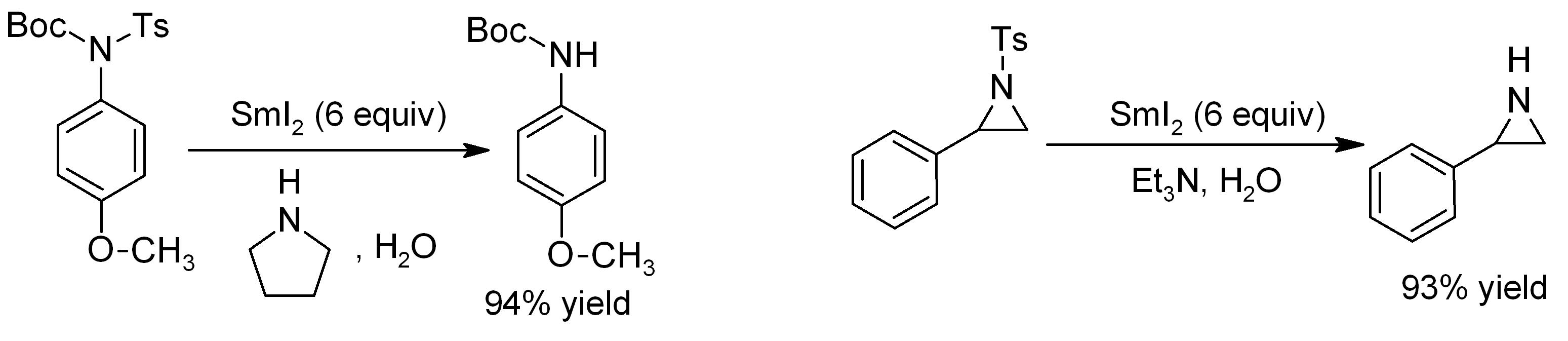
1,2-Diiodoethane is an organoiodine compound. Preparation and reactions 1,2-Diiodoethane can be prepared by the reaction of ethylene with iodine (I): :CH + I CHI 1,2-Diiodoethane is most commonly used in organic synthesis in the preparation of samarium(II) iodide Samarium(II) iodide is an inorganic compound with the formula SmI2. When employed as a solution for organic synthesis, it is known as Kagan's reagent. SmI2 is a green solid and forms a dark blue solution in THF. It is a strong one-electron re ... or ytterbium(II) iodide in an inert solvent such as THF. :Sm + ICH2CH2I → SmI2 + H2C=CH2 Spectral properties In mass spectroscopy, 1,2-diiodoethane exhibits 5 major peaks, with the base peak showing at 155 m/z, which is the loss of one iodine atom (127 m/z). References {{DEFAULTSORT:Diiodoethane, 1,2- Iodoalkanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. It is an isomer of another solvent, butanone. Production About 200,000 tonnes of tetrahydrofuran are produced annually. The most widely used industrial process involves the acid-catalyzed dehydration of 1,4-Butanediol, 1,4-butanediol. Ashland Inc., Ashland/ISP is one of the biggest producers of this chemical route. The method is similar to the production of diethyl ether from ethanol. The butanediol is derived from Condensation reaction, condensation of acetylene with formaldehyde followed by hydrogenation. DuPont developed a process for producing THF by oxidizing Butane#Isomers, ''n''-butane to crude maleic anhydride, follow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gmelins Handbuch Der Anorganischen Chemie
The Gmelin database is a large database of organometallic and inorganic compounds updated quarterly. It is based on the German publication ''Gmelins Handbuch der anorganischen Chemie'' ("Gmelin's Handbook of Inorganic Chemistry") which was originally published by Leopold Gmelin in 1817;''Brockhaus ABC Chemie'', VEB F. A. Brockhaus Verlag Leipzig 1965, pp. 497–498. the last print edition, the 8th, appeared in the 1990s. Although published over many decades, the printed series was not uniform in coverage or currency. Some elements are represented only by decades-old and not updated slim summary volumes. Others (Fe, B, S, F, U, etc.) have numerous supplements. Most later supplement volumes focused on an element's organic complexes. Each volume lists its literature coverage date. The database currently contains every compound/reaction discovered between 1772 and 1995, amounting to 1.5 million compounds and 1.3 million different reactions, with over 85,000 titles, keywords and ab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium(II) Iodide
Samarium(II) iodide is an inorganic compound with the formula SmI2. When employed as a solution for organic synthesis, it is known as Kagan's reagent. SmI2 is a green solid and forms a dark blue solution in THF. It is a strong one-electron reducing agent that is used in organic synthesis. Structure In solid samarium(II) iodide, the metal centers are seven-coordinate with a face-capped octahedral geometry. In its ether adducts, samarium remains heptacoordinate with five ether and two terminal iodide ligands. Preparation Samarium iodide is easily prepared in nearly quantitative yields from samarium metal and either diiodomethane or 1,2-diiodoethane. When prepared in this way, its solutions is most often used without purification of the inorganic reagent. Solid, solvent-free SmI2 forms by high temperature decomposition of samarium(III) iodide (SmI3).G. Jantsch, N. Skalla: "Zur Kenntnis der Halogenide der seltenen Erden. IV. – Über Samarium(II)jodid und den thermischen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ytterbium(II) Compounds
Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density, melting point and boiling point are much lower than those of most other lanthanides. In 1878, Swiss chemist Jean Charles Galissard de Marignac separated from the rare earth "erbia", another independent component, which he called "ytterbia", for Ytterby, the village in Sweden near where he found the new component of erbium. He suspected that ytterbia was a compound of a new element that he called "ytterbium". Four elements were named after the village, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodides
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is not. The low ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |