|
Yeast Flocculation
Yeast flocculation typically refers to the reversible clumping together (flocculation) of brewing yeast once the sugar in a wort has been fermented into beer. In the case of "top-fermenting" ale yeast (''Saccharomyces cerevisiae''), the yeast creates a krausen, or barm on the top of the liquid, unlike "bottom-fermenting" lager yeast (''Saccharomyces pastorianus'') where the yeast falls to the bottom of the brewing vessel. Process Cell aggregation occurs throughout microbiology, in bacteria, filamentous algae, fungi and yeast. Yeast are capable of forming three aggregates; mating aggregates, for DNA exchange; chain formation; and flocs as a survival strategy in adverse conditions. Industrial brewing strains rarely mate. Therefore, only chain formation and flocculation are of relevance to the brewing industry. Yeast flocculation is distinct from agglomeration (‘grit’ formation), which is irreversible and occurs most commonly in baker's yeast when strains fail to separate when res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flocculation
In colloidal chemistry, flocculation is a process by which colloidal particles come out of Suspension (chemistry), suspension to sediment in the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from Precipitation (chemistry), precipitation in that, prior to flocculation, colloids are merely suspended, under the form of a stable dispersion (where the internal phase (solid) is dispersed throughout the external phase (fluid) through mechanical agitation) and are not truly dissolved in Solution (chemistry), solution. Coagulation (water treatment), Coagulation and flocculation are important processes in fermentation and water treatment with coagulation aimed to destabilize and aggregate particles through chemical interactions between the coagulant and colloids, and flocculation to sediment the destabilized particles by causing their aggregation into floc. Term definition According to the IUPAC definition, flocculation is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EDTA
Ethylenediaminetetraacetic acid (EDTA), also called EDTA acid, is an aminopolycarboxylic acid with the formula . This white, slightly water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes even at neutral pH. It is thus used to dissolve Fe- and Ca-containing scale as well as to deliver iron ions under conditions where its oxides are insoluble. EDTA is available as several salts, notably disodium EDTA, sodium calcium edetate, and tetrasodium EDTA, but these all function similarly. Uses EDTA is widely used in industry. It also has applications in food preservation, medicine, cosmetics, water softening, in laboratories, and other fields. Industrial EDTA is mainly used to sequester (bind or confine) metal ions in aqueous solution. In the textile industry, it prevents metal ion impurities from modifying colours of dyed products. In the pulp and paper industry, EDTA inhibits the ability of metal ions, especiall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltose
} Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar. History Maltose was discovered by Augustin-Pierre Dubrunfaut, although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer Cornelius O'Sullivan. Its name comes from malt, combined with the suffix ' -ose' which is used in names of sugars. Structure and nomenclature Carbohydrates are generally divided into monosaccharides, oligosaccharides, and polysaccharides depending on th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight. It is used by plants to make cellulose, the most abundant carbohydrate in the world, for use in cell walls, and by all living Organism, organisms to make adenosine triphosphate (ATP), which is used by the cell as energy. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as amylose and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form is -glucose, while its Stereoisomerism, stereoisomer L-glucose, -glucose is produced synthetically in comparatively small amounts and is less biologicall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mannose
Mannose is a sugar with the formula , which sometimes is abbreviated Man. It is one of the monomers of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism. Mannose is not an essential nutrient; it can be produced in the human body from glucose, or converted into glucose. Mannose provides 2–5 kcal/g. It is partially excreted in the urine. Etymology The root of both "mannose" and " mannitol" is manna, which the Bible describes as the food supplied to the Israelites during their journey in the region of Sinai. Several trees and shrubs can produce a substance called manna, such as the "manna tree" (''Fraxinus ornus'') from whose secretions mannitol was originally isolated. Structure Mannose commonly exists as two different-sized rings, the py ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schizosaccharomyces
''Schizosaccharomyces'' is a genus of fission yeasts. The most well-studied species is '' S. pombe''. At present five Schizosaccharomyces species have been described ('' S. pombe'', ''S. japonicus'', ''S. octosporus'', ''S. cryophilus'' and ''S. osmophilus''). Like the distantly related ''Saccharomyces cerevisiae'', ''S. pombe'' is a significant model organism in the study of eukaryotic cell biology. It is particularly useful in evolutionary studies because it is thought to have diverged from the ''Saccharomyces cerevisiae ''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungal microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have be ...'' lineage between 300 million and 1 billion years ago, and thus provides an evolutionarily distant comparison. See also * Yeast in winemaking References External linksdiArk - Schizosaccharomyces {{Authorit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kluyveromyces
''Kluyveromyces'' is a genus of ascomycetous yeasts in the family Saccharomycetaceae. Some of the species, such as ''Kluyveromyces marxianus, K. marxianus'', are the teleomorphs of ''Candida (genus), Candida species''. The genus name of ''Kluyveromyces'' is in honour of Albert Jan Kluyver Royal Society, ForMemRS (1888-1956), who was a Dutch people, Dutch microbiologist and biochemist. The genus was circumscription (taxonomy), circumscribed by Johannes P. Van der Walt in Antonie van Leeuwenhoek (journal), Antonie van Leeuwenhoek vol.22 on pages 268–271 in 1956. Mating and spore, sporulation in ''Kluyveromyces'' are co-induced by poor environments and most often occur in succession without intervening ploidy, diploid mitosis, mitotic cell divisions.Hanson SJ, Wolfe KH. An Evolutionary Perspective on Yeast Mating-Type Switching. Genetics. 2017 May;206(1):9-32. doi: 10.1534/genetics.117.202036. [//www.ncbi.nlm.nih.gov/pubmed/28476860?dopt=Abstract PMID 28476860]; PMCID: PMC5419495 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lectin
Lectins are carbohydrate-binding proteins that are highly specific for sugar Moiety (chemistry), groups that are part of other molecules, so cause agglutination (biology), agglutination of particular cells or precipitation of glycoconjugates and polysaccharides. Lectins have a role in recognition at the cellular and molecular level and play numerous roles in biological recognition phenomena involving cells, carbohydrates, and proteins. Lectins also mediate attachment and binding of bacteria, viruses, and fungi to their intended targets. Lectins are found in many foods. Some foods, such as beans and grains, need to be cooked, fermented or sprouted to reduce lectin content. Some lectins are beneficial, such as CLEC11A, which promotes bone growth, while others may be powerful toxins such as ricin. Lectins may be disabled by specific monosaccharides, mono- and oligosaccharides, which bind to ingested lectins from grains, legumes, nightshade plants, and dairy; binding can prevent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mannan (polysaccharide)
Mannans are polymers containing the sugar mannose as a principal component. They are a type of polysaccharide found in hemicellulose, a major source of biomass found in higher plants such as softwoods. These polymers also typically contain two other sugars, galactose and glucose. They are often branched (unlike cellulose). Structural diversity Plant mannans have β(1-4) linkages, occasionally with α(1-6) galactose branches, forming galactomannans. They are insoluble and a form of storage polysaccharide. Ivory nut is a source of mannans. An additional type is galactoglucomannan found in soft wood with a mixed mannose/glucose β(1-4) backbone. Many mannans are acetylated and some from marine sources, have sulfate esters side chains. Yeast and some plants such as conjac and salep have a different type of mannans in their cell wall, with a α(1-6) linked backbone and α(1-2) and α(1-3) linked glucose branches, hence "glucomannan". It is water soluble. It is serologically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
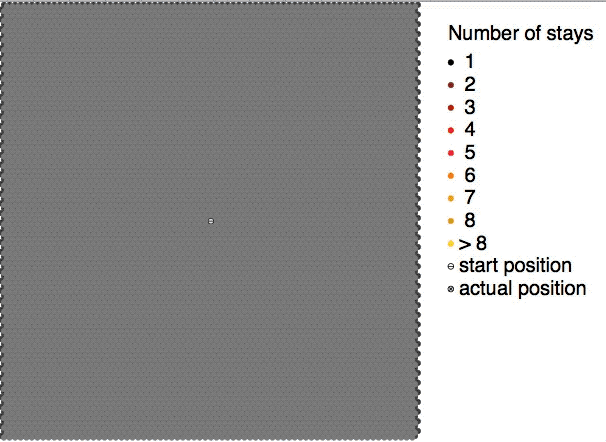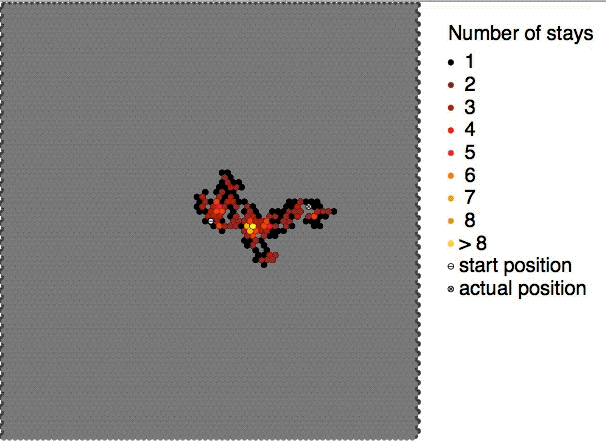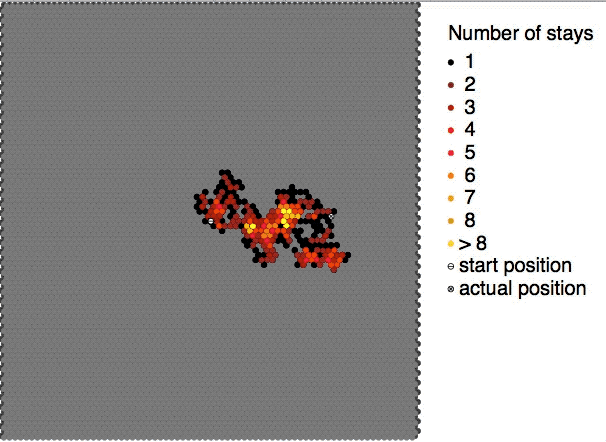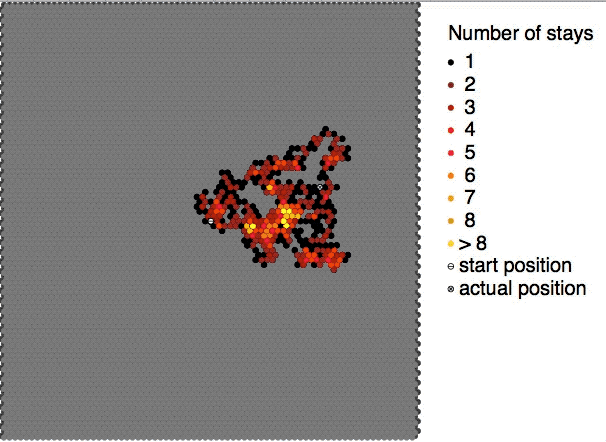
Brownian Motion
Brownian motion is the random motion of particles suspended in a medium (a liquid or a gas). The traditional mathematical formulation of Brownian motion is that of the Wiener process, which is often called Brownian motion, even in mathematical sources. This motion pattern typically consists of Randomness, random fluctuations in a particle's position inside a fluid sub-domain, followed by a relocation to another sub-domain. Each relocation is followed by more fluctuations within the new closed volume. This pattern describes a fluid at thermal equilibrium, defined by a given temperature. Within such a fluid, there exists no preferential direction of flow (as in transport phenomena). More specifically, the fluid's overall Linear momentum, linear and Angular momentum, angular momenta remain null over time. The Kinetic energy, kinetic energies of the molecular Brownian motions, together with those of molecular rotations and vibrations, sum up to the caloric component of a fluid's in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobe
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. The term ''hydrophobic''—which comes from the Ancient Greek (), "having a fear of water", constructed Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon. revised and augmented throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |