|
Whiteite
Whiteite is a rare hydrated hydroxyphosphate mineral. Whiteite subgroup The name whiteite refers to three minerals in the jahnsite-whiteite group, whiteite subgroup. Subgroup members (formulae from IMA): *Whiteite-(CaFeMg), IMA1975-001, CaFe2+Mg2Al2(PO4)4(OH)2·8H2O *Whiteite-(MnFeMg), IMA1978-A, Mn2+Fe2+Mg2Al2(PO4)4(OH)2·8H2O *Whiteite-(CaMnMg), IMA1986-012, CaMn2+Mg2Al2(PO4)4(OH)2·8H2O *Rittmannite, Mn2+Mn2+Fe2+2Al2(PO4)4(OH)2·8H2O In the whiteite formulae, the symbols in brackets indicate the dominant atom in three distinct structural positions, designated X, M(1), and M(2), in that order; for instance, magnesium Mg is always the dominant atom in the M(2) position for all the whiteite minerals. Whiteite was named after John Sampson White Jr (born 1933), associate curator of minerals at the Smithsonian Institution, and founder, editor and publisher (1970–1982) of the Mineralogical Record. Unit cell All members of the series belong to the monoclinic crystal system with p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
Phosphates are the naturally occurring form of the element phosphorus. In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, phosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one proton gives the dihydrogen phosphate ion while removal of two protons gives the hydrogen phosphate ion . These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate or orthophosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Birefringence
Birefringence, also called double refraction, is the optical property of a material having a refractive index that depends on the polarization and propagation direction of light. These optically anisotropic materials are described as birefringent or birefractive. The birefringence is often quantified as the maximum difference between refractive indices exhibited by the material. Crystals with non-cubic crystal structures are often birefringent, as are plastics under mechanical stress. Birefringence is responsible for the phenomenon of double refraction whereby a ray of light, when incident upon a birefringent material, is split by polarization into two rays taking slightly different paths. This effect was first described by Danish scientist Rasmus Bartholin in 1669, who observed it in Iceland spar (calcite) crystals which have one of the strongest birefringences. In the 19th century Augustin-Jean Fresnel described the phenomenon in terms of polarization, understanding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
École Nationale Supérieure Des Mines De Paris
École or Ecole may refer to: * an elementary school in the French educational stages normally followed by secondary education establishments (collège and lycée) * École (river), a tributary of the Seine flowing in région Île-de-France * École, Savoie, a French commune * École-Valentin, a French commune in the Doubs département * Grandes écoles, higher education establishments in France * The École The École, formerly Ecole Internationale de New York, is an intimate and independent French-American school, which cultivates an internationally minded community of students from 2 to 14 years old in New York City’s vibrant Flatiron Distric ..., a French-American bilingual school in New York City * Ecole Software, a Japanese video-games developer/publisher {{disambiguation, geo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Custer County, South Dakota
Custer County is a county in the U.S. state of South Dakota. As of the 2020 census, the population was 8,318. Its county seat is Custer. The county was created in 1875, and was organized in 1877. It was named after General George Armstrong Custer. Custer County is home to two of the three longest caves in the United States: Jewel Cave National Monument and Wind Cave National Park. Geography Custer County lies on the west line of South Dakota. Its west boundary line abuts the east boundary line of the state of Wyoming. The Cheyenne River flows northeastward along the upper portion of the county's east boundary. Battle Creek flows southeastward in the upper eastern part of the county, discharging into Cheyenne River along the county's northeastern boundary line. Spring Creek flows northeastward through the upper eastern part of the county, discharging into the river just north of the county border. The county terrain is mountainous, especially its western portion. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Taquaral
Taquaral is a municipality in the state of São Paulo in Brazil. The population is 2,819 (2015 est.) in an area of 53.9 km2. The elevation is 639 m. Media In telecommunications, the city was served by Telecomunicações de São Paulo. In July 1998, this company was acquired by Telefónica, which adopted the Vivo brand in 2012. The company is currently an operator of cell phones, fixed lines, internet (fiber optics/4G) and television (satellite and cable). Religion Christianity is present in the city as follows: Catholic Church The Catholic church in the municipality is part of the Roman Catholic Diocese of Jaboticabal. Protestant Church The most diverse evangelical beliefs are present in the city, mainly Pentecostal, including the Assemblies of God in Brazil (the largest evangelical church in the country), Christian Congregation in Brazil, among others. These denominations are growing more and more throughout Brazil. See also * List of municipalities in São ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type Locality (geology)
Type locality, also called type area, is the locality where a particular rock type, stratigraphic unit or mineral species is first identified. If the stratigraphic unit in a locality is layered, it is called a stratotype, whereas the standard of reference for unlayered rocks is the type locality. The concept is similar to type site in archaeology. Examples of geological type localities Rocks and minerals * Aragonite: Molina de Aragón, Guadalajara, Spain * Autunite: Autun, France * Benmoreite: Ben More (Mull), Scotland * Blairmorite: Blairmore, Alberta, Canada * Boninite: Bonin Islands, Japan * Comendite: Comende, San Pietro Island, Sardinia * Cummingtonite: Cummington, Massachusetts * Dunite: Dun Mountain, New Zealand * Essexite: Essex County, Massachusetts, US * Fayalite: Horta, Fayal Island, Azores, Portugal * Harzburgite: Bad Harzburg, Germany * Icelandite: Thingmuli (Þingmúli), Iceland * Ijolite: Iivaara, Kuusamo, Finland * Kimberlite: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Gravity
Relative density, also called specific gravity, is a dimensionless quantity defined as the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for solids and liquids is nearly always measured with respect to water at its densest (at ); for gases, the reference is air at room temperature (). The term "relative density" (abbreviated r.d. or RD) is preferred in SI, whereas the term "specific gravity" is gradually being abandoned. If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
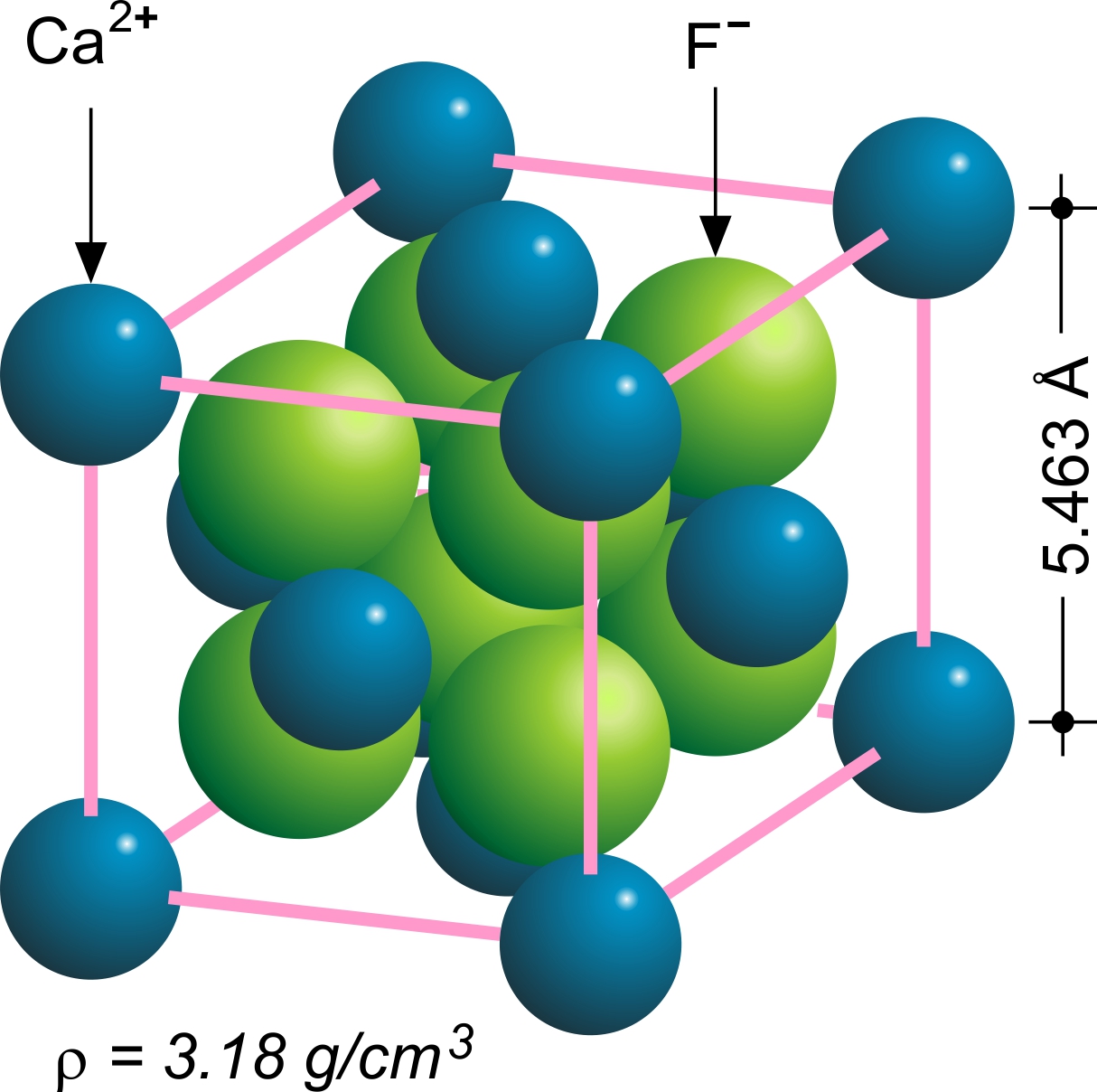
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite has anomalous partial dispersion, that is, its refractive index varies with the wavelength of light in a manner that differs from that of commonly used glasses, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on Scratch hardness, scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German , a term from the 19th century that came from the Latin word for Lime (material), lime, (genitive ) with the suffix ''-ite'' used to name minerals. It is thus a Doublet (linguistics), doublet of the word ''wikt:chalk, chalk''. When applied by archaeology, archaeologists and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mohs Hardness
The Mohs scale ( ) of mineral hardness is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of mineral In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Mi ...s through the ability of harder material to scratch softer material. The scale was introduced in 1812 by the German geologist and Mineralogy, mineralogist Friedrich Mohs, in his book (English: Attempt at an elementary method for the natural-historical determination and recognition of fossils); it is one of several definitions of hardness comparison, hardness in materials science, some of which are more quantitative. The method of comparing hardness by observing which minerals can scratch others is of great antiquity, having been mentioned by Theophrastus in his treatise ''On Stones'', , followed by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cleavage (crystal)
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye. If bonds in certain directions are weaker than others, the crystal will tend to split along the weakly bonded planes. These flat breaks are termed "cleavage".Hurlbut, Cornelius S.; Klein, Cornelis, 1985, '' Manual of Mineralogy'', 20th ed., Wiley, The classic example of cleavage is mica, which cleaves in a single direction along the basal pinacoid, making the layers seem like pages in a book. In fact, mineralogists often refer to "books of mica". Diamond and graphite provide examples of cleavage. Each is composed solely of a single element, carbon. In diamond, each carbon atom is bonded to four others in a tetrahedral patter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic Crystal System
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal In mathematics, orthogonality (mathematics), orthogonality is the generalization of the geometric notion of ''perpendicularity''. Although many authors use the two terms ''perpendicular'' and ''orthogonal'' interchangeably, the term ''perpendic .... Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , ro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |