|
Volumetric Heat Capacity
The volumetric heat capacity of a material is the heat capacity of a sample of the substance divided by the volume of the sample. It is the amount of energy that must be added, in the form of heat, to one unit of volume of the material in order to cause an increase of one unit in its temperature. The SI unit of volumetric heat capacity is joule per kelvin per cubic meter, J⋅K−1⋅m−3. The volumetric heat capacity can also be expressed as the specific heat capacity (heat capacity per unit of mass, in J⋅K−1⋅ kg−1) times the density of the substance (in kg/ L, or g/ mL). It is defined to serve as an intensive property. This quantity may be convenient for materials that are commonly measured by volume rather than mass, as is often the case in engineering and other technical disciplines. The volumetric heat capacity often varies with temperature, and is different for each state of matter. While the substance is undergoing a phase transition, such as melting or bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K). Heat capacity is an extensive property. The corresponding intensive property is the specific heat capacity, found by dividing the heat capacity of an object by its mass. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The volumetric heat capacity measures the heat capacity per volume. In architecture and civil engineering, the heat capacity of a building is often referred to as its '' thermal mass''. Definition Basic definition The heat capacity of an object, denoted by C, is the limit C = \lim_\frac, where \Delta Q is the amount of heat that must be added to the object (of mass ''M'') in order to raise its temperature by \Delta T. The value of this parameter usually varies considerably depending o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Granite
Granite ( ) is a coarse-grained (phanerite, phaneritic) intrusive rock, intrusive igneous rock composed mostly of quartz, alkali feldspar, and plagioclase. It forms from magma with a high content of silica and alkali metal oxides that slowly cools and solidifies underground. It is common in the continental crust of Earth, where it is found in igneous intrusions. These range in size from dike (geology), dikes only a few centimeters across to batholiths exposed over hundreds of square kilometers. Granite is typical of a larger family of ''granitic rocks'', or ''granitoids'', that are composed mostly of coarse-grained quartz and feldspars in varying proportions. These rocks are classified by the relative percentages of quartz, alkali feldspar, and plagioclase (the QAPF diagram, QAPF classification), with true granite representing granitic rocks rich in quartz and alkali feldspar. Most granitic rocks also contain mica or amphibole minerals, though a few (known as leucogranites) conta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octane
Octane is a hydrocarbon and also an alkane with the chemical formula C8H18, and the condensed structural formula CH3(CH2)6CH3. Octane has many structural isomers that differ by the location of branching in the carbon chain. One of these isomers, 2,2,4-Trimethylpentane, 2,2,4-trimethylpentane (commonly called iso-octane), is used as one of the standard values in the octane rating scale. Octane is a component of gasoline and petroleum. Under standard temperature and pressure, octane is an odorless, colorless liquid. Like other short-chained alkanes with a low molecular weight, it is Volatility (chemistry), volatile, flammable, and toxic. Octane is 1.2 to 2 times more toxic than heptane. Isomers N-octane has 23 Structural isomers, constitutional isomers. 8 of these isomers have one stereocenter; 3 of them have two stereocenters. Achiral isomers: * 2-Methylheptane * 4-Methylheptane * 3-Ethylhexane * 2,2-Dimethylhexane * 2,5-Dimethylhexane * 3,4-Dimethylhexane, (''meso'')-3,4-Dime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs naturally, and its sulfide and oxide forms are important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery-white color when freshly produced. Surface oxidation generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly iridescent appearance due to thin-film interference. Bismuth is both the most diamagnetic element and one of the least thermally conductive metals known. Bismuth was formerly understood to be the element with the highest atomic mass whose nuclei do not spontaneously decay. However, in 2003 it was found to be very slightly radioactive. The metal's only primordial isotope, bismuth-209, undergoes alpha decay with a half-l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Mass
In chemistry, the molar mass () (sometimes called molecular weight or formula weight, but see related quantities for usage) of a chemical substance ( element or compound) is defined as the ratio between the mass () and the amount of substance (, measured in moles) of any sample of the substance: . The molar mass is a bulk, not molecular, property of a substance. The molar mass is a ''weighted'' ''average'' of many instances of the element or compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molecular mass (for molecular compounds) and formula mass (for non-molecular compounds, such as ionic salts) are commonly used as synonyms of molar mass, as the numerical values are identical (for all practical purposes), differing only in units ( dalton vs. g/mol o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Weight
Relative atomic mass (symbol: ''A''; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant. The atomic mass constant (symbol: ''m'') is defined as being of the mass of a carbon-12 atom. Since both quantities in the ratio are masses, the resulting value is dimensionless. These definitions remain valid even after the 2019 revision of the SI. For a single given sample, the relative atomic mass of a given element is the weighted arithmetic mean of the masses of the individual atoms (including all its isotopes) that are present in the sample. This quantity can vary significantly between samples because the sample's origin (and therefore its radioactive history or diffusion history) may have produced combinations of isotopic abundances in varying ratios. For example, due to a different mixture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
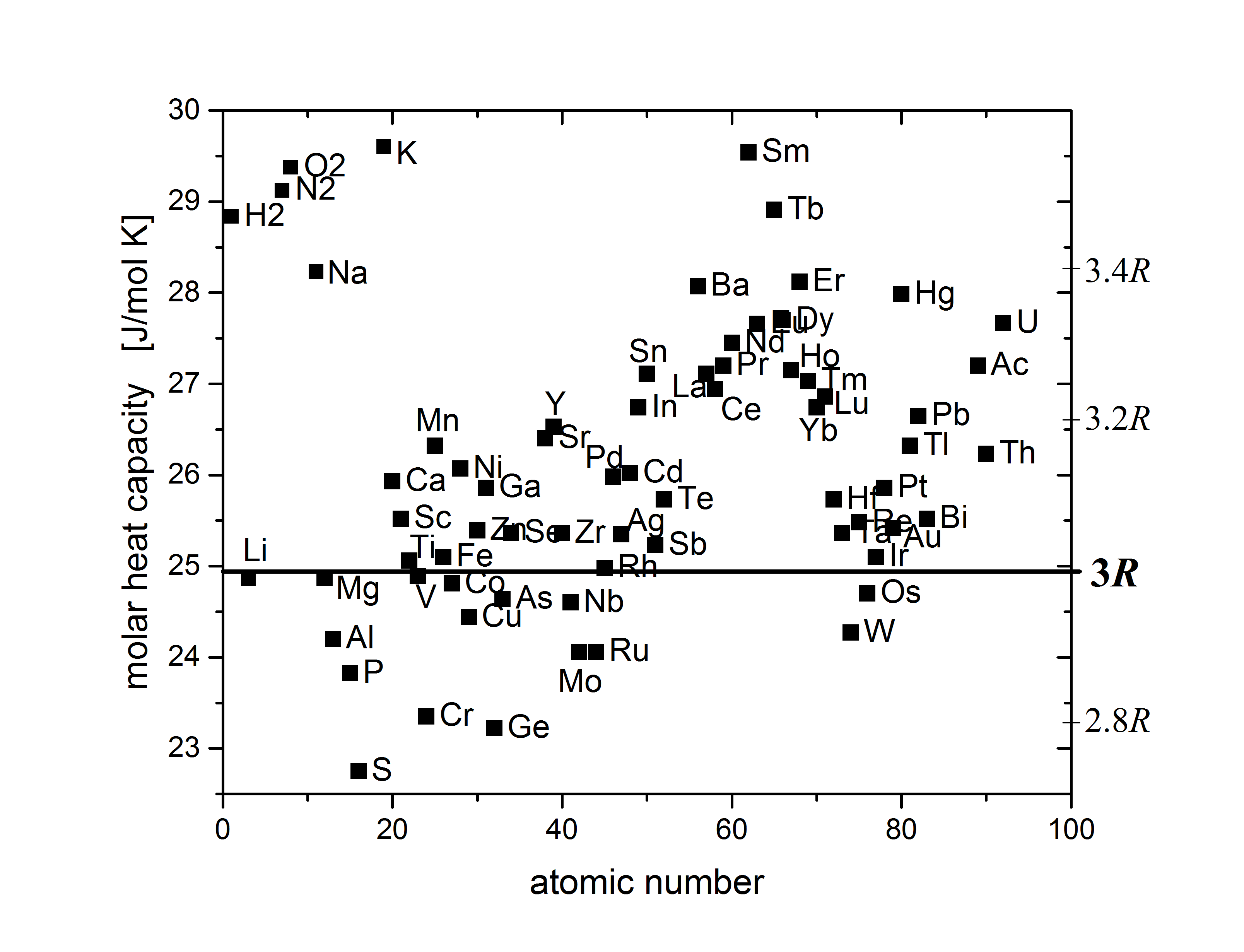
Dulong–Petit Law
The Dulong–Petit law, a thermodynamic law proposed by French physicists Pierre Louis Dulong and Alexis Thérèse Petit, states that the classical expression for the molar specific heat capacity of certain chemical elements is constant for temperatures far from the absolute zero. In modern terms, Dulong and Petit found that the heat capacity of a mole (unit), mole of many solid elements is about 3''R'', where ''R'' is the universal gas constant. The modern theory of the heat capacity of solids states that it is due to phonon, lattice vibrations in the solid. History Experimentally Pierre Louis Dulong and Alexis Thérèse Petit had found in 1819 that the heat capacity per weight (the mass-specific heat capacity) for 13 measured elements was close to a constant value, after it had been multiplied by a number representing the presumed relative atomic weight of the element. These atomic weights had shortly before been suggested by John Dalton and modified by Jacob Berzelius. Du ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alexis Thérèse Petit
Alexis Thérèse Petit (; 2 October 1791 – 21 June 1820) was a French physicist. Petit is known for his work on the efficiencies of air- and steam-engines, published in 1818 (''Mémoire sur l’emploi du principe des forces vives dans le calcul des machines''). His well-known discussions with the French physicist Sadi Carnot, founder of thermodynamics, may have stimulated Carnot in his reflexions on heat engines and thermodynamic efficiency. The Dulong–Petit law (1819) is named after him and his collaborator Pierre Louis Dulong. Biography Early life and studies Petit was born in Vesoul, Haute-Saône. At the age of 10, he proved that he was already capable of taking the difficult entrance exam to France's most prestigious scientific school of the time, the École polytechnique of Paris. He was then placed in a preparatory school where he actually served as a "''répétiteur"'' to help his own classmates digest the course material. He duly entered the Polytechnique at the l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pierre Louis Dulong
Pierre Louis Dulong FRS FRSE (; ; 12 February 1785 – 19 July 1838) was a French physicist and chemist. He is remembered today largely for the law of Dulong and Petit, although he was much-lauded by his contemporaries for his studies into the elasticity of steam, conduction of heat, and specific heats of gases. He worked most extensively on the specific heat capacity and the expansion and refractive indices of gases. He collaborated the co-discoverer of the Dulong–Petit law. Early life and education Dulong was born in Rouen, France. An only child, he was orphaned at the age of 4, he was brought up by his aunt in Auxerre. He gained his secondary education in Auxerre and the Lycée Pierre Corneille in Rouen before entering the École polytechnique, Paris in 1801, only for his studies to be impeded by poor health. He began studying medicine, but gave this up, possibly because of a lack of financial means, to concentrate on science, working under the direction of Louis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Heat Capacity
The molar heat capacity of a chemical substance is the amount of energy that must be added, in the form of heat, to one mole (unit), mole of the substance in order to cause an increase of one unit in its temperature. Alternatively, it is the heat capacity of a sample of the substance divided by the amount of substance of the sample; or also the specific heat capacity of the substance times its molar mass. The International System of Units, SI unit of molar heat capacity is joule per kelvin per Mole (unit), mole, J⋅K−1⋅mol−1. Like the specific heat, the measured molar heat capacity of a substance, especially a gas, may be significantly higher when the sample is allowed to expand as it is heated (at constant pressure, or isobaric) than when it is heated in a closed vessel that prevents expansion (at constant volume, or isochoric). The ratio between the two, however, is the same heat capacity ratio obtained from the corresponding specific heat capacities. This property is m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mole (unit)
The mole (symbol mol) is a unit of measurement, the base unit in the International System of Units (SI) for ''amount of substance'', an SI base quantity proportional to the number of elementary entities of a substance. One mole is an aggregate of exactly elementary entities (approximately 602 sextillion or 602 billion times a trillion), which can be atoms, molecules, ions, ion pairs, or other particles. The number of particles in a mole is the Avogadro number (symbol ) and the numerical value of the '' Avogadro constant'' (symbol ) expressed in mol−1. The relationship between the mole, Avogadro number, and Avogadro constant can be expressed in the following equation:1\text = \frac = \frac The current SI value of the mole is based on the historical definition of the mole as the amount of substance that corresponds to the number of atoms in 12 grams of 12C, which made the molar mass of a compound in grams per mole, numerically equal to the average molecular mass or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Capacity Ratio
In thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or Laplace's coefficient, is the ratio of the heat capacity at constant pressure () to heat capacity at constant volume (). It is sometimes also known as the '' isentropic expansion factor'' and is denoted by (gamma) for an ideal gasγ first appeared in an article by the French mathematician, engineer, and physicist Siméon Denis Poisson: * On p. 332, Poisson defines γ merely as a small deviation from equilibrium which causes small variations of the equilibrium value of the density ρ. In Poisson's article of 1823 – * γ was expressed as a function of density D (p. 8) or of pressure P (p. 9). Meanwhile, in 1816 the French mathematician and physicist Pierre-Simon Laplace had found that the speed of sound depends on the ratio of the specific heats. * However, he didn't denote the ratio as γ. In 1825, Laplace stated that the speed of sound i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |