|
Vanadium(II) Iodide
Vanadium(II) iodide is the inorganic compound with the formula VI2. It is a black micaceous solid. It adopts the cadmium iodide structure, featuring octahedral V(II) centers. The hexahydrate ()62, an aquo complex, is also known. It forms red-violet crystals. The hexahydrate dehydrates under vacuum to give a red-brown tetrahydrate with the formula V()4I2. Preparation The original synthesis of VI2 involved reaction of the elements. Solvated vanadium(II) iodides can be prepared by reduction of vanadium(III) chlorides with trimethylsilyl iodide Trimethylsilyl iodide (iodotrimethylsilane or TMSI) is an organosilicon compound with the chemical formula (CH3)3SiI. It is a colorless, volatile liquid at room temperature. Preparation Trimethylsilyl iodide may be prepared by the oxidative cleav .... It reacts with anhydrous ammonia to give the hexaammine complex. References {{Iodides Vanadium(II) compounds Iodides Metal halides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium(II) Chloride
Vanadium(II) chloride is the inorganic compound with the formula VCl2, and is the most reduced vanadium chloride. Vanadium(II) chloride is an apple-green solid that dissolves in water to give purple solutions. Preparation, properties, and related compounds Solid VCl2 is prepared by thermal decomposition of VCl3, which leaves a residue of VCl2:Young, R. C.; Smith, M. E. "Vanadium(II) Chloride" Inorganic Syntheses, 1953, volume IV, page 126-127. :2 VCl3 → VCl2 + VCl4 VCl2 dissolves in water to give the purple hexaaquo ion (H2O)6sup>2+. Evaporation of such solutions produces crystals of (H2O)6l2. Vanadium dichloride is used as a specialty reductant in organic chemistry. As an aqueous solution, it converts cyclohexylnitrate to cyclohexanone. It reduces phenyl azide into aniline Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine In organic chemistry, an aromatic amine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium(II) Bromide
Vanadium(II) bromide is a inorganic compound with the formula VBr2. It adopts the cadmium iodide structure, featuring octahedral V(II) centers. A hexahydrate is also known. The hexahydrate undergoes partial dehydration to give the tetrahydrate. Both the hexa- and tetrahydrates are bluish in color. The compound is produced by the reduction of vanadium(III) bromide with hydrogen Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic .... Further reading *Stebler, A.; Leuenberger, B.; Guedel, H. U. "Synthesis and crystal growth of A3M2X9 (A = Cs, Rb; M = Ti, V, Cr; X = Cl, Br)" Inorganic Syntheses (1989), volume 26, pages 377–85. References {{Bromides Bromides Metal halides Vanadium(II) compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
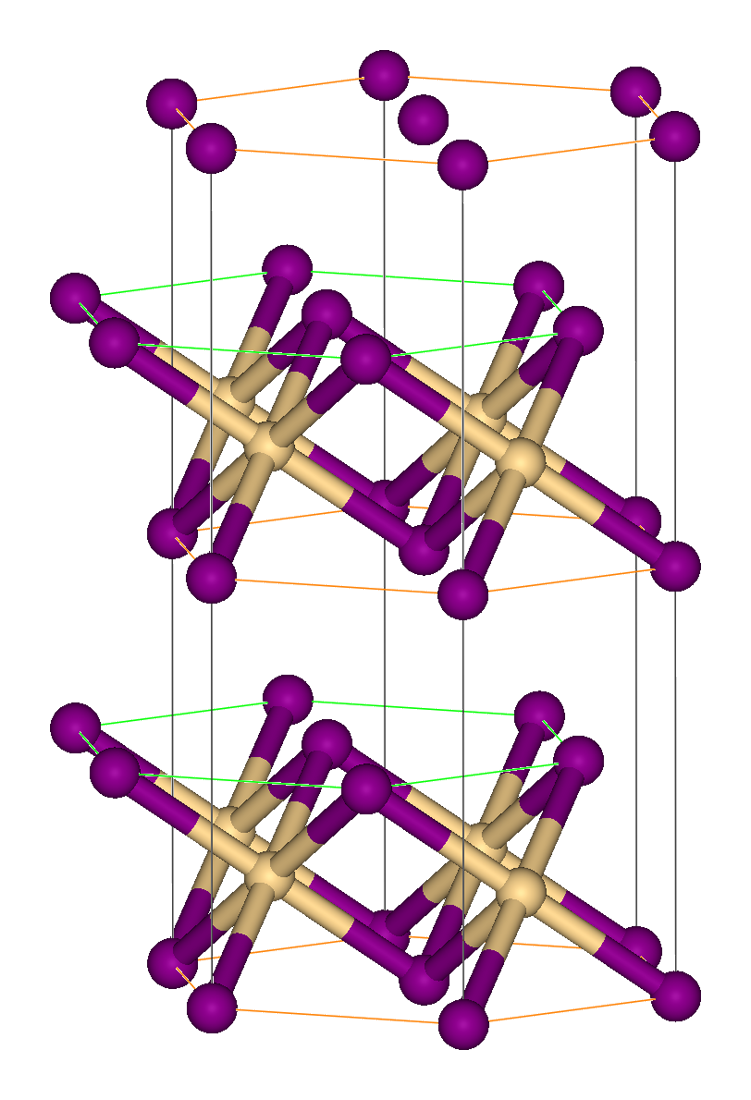
Vanadium(III) Iodide
Vanadium(III) iodide is the inorganic compound with the formula VI3. This paramagnetic solid is generated by the reaction of vanadium powder with iodine at around 500 °C. The black hygroscopic crystals dissolve in water to give green solutions, characteristic of V(III) ions. The purification of vanadium metal by the chemical transport reaction involving the reversible formation of vanadium(III) iodides in the presence of iodine and its subsequent decomposition to yield pure metal: : 2 V + 3 I2 ⇌ 2 VI3 VI3 crystallizes in the motif adopted by bismuth(III) iodide Bismuth(III) iodide is the inorganic compound with the formula Bi I3. This gray-black salt is the product of the reaction of bismuth and iodine, which once was of interest in qualitative inorganic analysis. Bismuth(III) iodide adopts a distin ...: the iodides are hexagonal-closest packed and the vanadium centers occupy one third of the octahedral holes. When solid samples are heated, the gas contains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon ( graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mica
Micas ( ) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into extremely thin elastic plates. This characteristic is described as perfect basal cleavage. Mica is common in igneous and metamorphic rock and is occasionally found as small flakes in sedimentary rock. It is particularly prominent in many granites, pegmatites, and schists, and "books" (large individual crystals) of mica several feet across have been found in some pegmatites. Micas are used in products such as drywalls, paints, fillers, especially in parts for automobiles, roofing and shingles, as well as in electronics. The mineral is used in cosmetics and food to add "shimmer" or "frost." Properties and structure The mica group is composed of 37 phyllosilicate minerals. All crystallize in the monoclinic system, with a tendency towards pseudohexagonal crystals, and are similar in structure but vary in chemical composition. Mic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Iodide
Cadmium iodide is the inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects. Preparation Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine. Crystal structure In cadmium iodide the iodide anions form a hexagonal close packed arrangement while the cadmium cations fill all of the octahedral sites in alternate layers. The resultant structure consists of a layered lattice. This same basic structure is found in many other salts and minerals. Cadmium iodide is mostly ionically bonded but with partial covalent character. Cadmium iodide's crystal structure is the prototype on which the crystal structures many other compounds can be conside ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aquo Complex
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry . Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand (" homoleptic aquo complexes"), but of course many complexes are known to consist of a mix of aquo and other ligands. Stoichiometry and structure Hexa-aquo complexes Most aquo complexes are mono-nuclear, with the general formula , with or 3; they have an octahedral structure. The water molecules function as Lewis bases, donating a pair of electrons to the metal ion and forming a dative covalent bond with it. Typical examples are listed in the following table. : Tutton's salts are crystalline compounds with the generic formula (wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium(III) Chloride
Vanadium trichloride is the inorganic compound with the formula VCl3. This purple salt is a common precursor to other vanadium(III) complexes. Structure VCl3 has the common BiI3 structure, a motif that features hexagonally closest-packed chloride framework with vanadium ions occupying the octahedral holes. VBr3 and VI3 adopt the same structure, but VF3 features a structure more closely related to ReO3. VCl3 is paramagnetic and has two unpaired electrons. Preparation and reactions VCl3 is prepared by heating VCl4 at 160–170 °C under a flowing stream of inert gas, which sweeps out the Cl2. The bright red liquid converts to a purple solid. Heating of VCl3 decomposes with volatilization of VCl4, leaving VCl2. Upon heating under H2 at 675 °C (but less than 700 °C), VCl3 reduces to greenish VCl2. :: 2 VCl3 + H2 → 2 VCl2 + 2 HCl Comproportionation of vanadium trichloride and vanadium(V) oxides gives vanadium oxydichloride: :V2O5 + VOCl3 + 3 VCl3 → ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilyl Iodide
Trimethylsilyl iodide (iodotrimethylsilane or TMSI) is an organosilicon compound with the chemical formula (CH3)3SiI. It is a colorless, volatile liquid at room temperature. Preparation Trimethylsilyl iodide may be prepared by the oxidative cleavage of hexamethyldisilane by iodine or by the cleavage of hexamethyldisiloxane with aluminium triiodide. : TMS-TMS + I2 → 2 TMSI (TMS = (CH3)3Si) : 3 TMS-O-TMS + 2 AlI3 → 6 TMSI + Al2O3 Applications Trimethylsilyl iodide is used to introduce the trimethylsilyl group onto alcohols (ROH): :R-OH + TMSI → R-OTMS + HI This type of reaction may be useful for gas chromatography analysis; the resultant silyl ether is more volatile than the underivatized original materials. However, for the preparation of bulk trimethylsilylated material, trimethylsilyl chloride may be preferred due to its lower cost. TMSI reacts with alkyl ether In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexaammine Complex
In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia () ligand. "Ammine" is spelled this way due to historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.A. von Zelewsky "Stereochemistry of Coordination Compounds" John Wiley: Chichester, 1995. . History Ammine complexes played a major role in the development of coordination chemistry, specifically determination of the stereochemistry and structure. They are easily prepared, and the metal-nitrogen ratio can be determined by elemental analysis. Through studies mainly on the ammine complexes, Alfred Werner developed his Nobel Prize-winning concept of the structure of coordination compounds (see Figure). One of the first ammine complexes to be described was Magnus' green ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium(II) Compounds
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( passivation) somewhat stabilizes the free metal against further oxidation. Spanish scientist Andrés Manuel del Río discovered compounds of vanadium in 1801 in Mexico by analyzing a new lead-bearing mineral he called "brown lead". Though he initially presumed its qualities were due to the presence of a new element, he was later erroneously convinced by French chemist Hippolyte Victor Collet-Descotils that the element was just chromium. Then in 1830, Nils Gabriel Sefström generated chlorides of vanadium, thus proving there was a new element, and named it "vanadium" after the Scandinavian goddess of beauty and fertility, Vanadís (Freyja). The name was based on the wide range of colors found in vanadium compounds. Del Rio's lead mineral was u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodides
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_ion_in_aqueous_solution.jpg)

-3D-balls.png)
