|
Trimethylsilyl Trifluoromethanesulfonate
Trimethylsilyl trifluoromethanesulfonate (TMSOTf) is an organosilicon compound with the formula . It is a colorless moisture-sensitive liquid. It is the trifluoromethanesulfonate derivative of trimethylsilyl. It is mainly used to activate ketones and aldehydes in organic synthesis. Reactions TMSOTf is quite sensitive toward hydrolysis: : It is far more electrophilic than trimethylsilyl chloride. Related to its tendency to hydrolyze, TMSOTf is effective for silylation of alcohols: : A common use of is for the preparation of silyl enol ethers. One example involves the synthesis of the silyl enol ether of camphor: : It was also used in Takahashi Taxol total synthesis and in chemical glycosylation reactions. Trimethylsilyl trifluoromethanesulfonate has a variety of other specialized uses. It has been used to install tert-alkyl groups on phosphine (R = alkyl): :PH3 + R3C–OAc + Me3SiOTf → R3C)2PH2Tf Deprotection of Boc-protected amines can be achieved using ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosilicon Compound
Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an ''inorganic'' compound. History In 1863, Charles Friedel and James Crafts made the first organochlorosilane compound. The same year, they also described a "polysilicic acid ether" in the preparation of ethyl- and methyl-o-silicic acid. Extensive research in the field of organosilicon compounds was pioneered in the beginning of 20th century by Frederic S. Kipping. He also had coined the term "silicone" (resembling ''ketones'', though this is erroneous) in relation to these materials in 1904. In recognition of Kipping's achievements, the Dow Chemical Company had established an award in the 1960s that is given for significant contributions to the field of silicon chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoromethanesulfonate
In organic chemistry, triflate (systematic name: trifluoromethanesulfonate), is a functional group with the formula and structure . The triflate group is often represented by , as opposed to −Tf, which is the triflyl group, . For example, ''n''-butyl triflate can be written as . The corresponding triflate anion, , is an extremely stable polyatomic ion; this comes from the fact that triflic acid () is a superacid; i.e. it is more acidic than pure sulfuric acid, already one of the strongest acids known. Applications A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution, Suzuki couplings and Heck reactions. Since alkyl triflates are extremely reactive in SN2 reactions, they must be stored in conditions free of nucleophiles (such as water). The anion owes its stability to resonance stabilization which causes the negative charge to be spread symmetrically over the three oxygen atoms. An additional stabilization i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si(CH3)3], which is in turn bonded to the rest of a molecule. This structural group is characterized by Chemically inert, chemical inertness and a large molecular volume, which makes it useful in a number of applications. A trimethylsilyl group bonded to a methyl group forms tetramethylsilane, which is abbreviated as TMS as well. Compounds with trimethylsilyl groups are not normally found in nature. Chemists sometimes use a trimethylsilylating reagent to derivatize rather non-volatile compounds such as certain Alcohol (chemistry), alcohols, phenols, or carboxylic acids by substituting a trimethylsilyl group for a hydrogen in the hydroxyl groups on the compounds. This way Silyl ether, trimethylsiloxy groups [−O-Si(CH3)3] are formed on the molecule. A couple of examples of trimethylsilylating agents include trimethyls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the general subject of organic synthesis, there are many different types of synthetic routes that can be completed including total synthesis, Enantioselective synthesis, stereoselective synthesis, automated synthesis, and many more. Additionally, in understanding organic synthesis it is necessary to be familiar with the methodology, techniques, and applications of the subject. Total synthesis A total synthesis refers to the complete chemical synthesis of molecules from simple, Precursor (chemistry), natural precursors. Total synthesis is accomplished either via a linear or convergent approach. In a Linear synthesis, ''linear'' synthesis—often adequate for simple structures—several steps are performed sequentially until the molecule is com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilyl Chloride
Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound ( silyl halide), with the formula , often abbreviated or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry. Preparation TMSCl is prepared on a large scale by the '' direct process'', the reaction of methyl chloride with a silicon-copper alloy. The principal target of this process is dimethyldichlorosilane, but substantial amounts of the trimethyl and monomethyl products are also obtained. The relevant reactions are (Me = methyl, ): x\ \ce \longrightarrow \begin \ce, \\ pt \ce, \\ pt \ce,\\ pt \text \end Typically about 2–4% of the product stream is the monochloride, which forms an azeotrope with . Reactions and uses TMSCl is reactive toward nucleophiles, resulting in the replacement of the chloride. In a characteristic reaction of TMSCl, the nucleophile is water, resulting in hydrolysis to give the hexa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
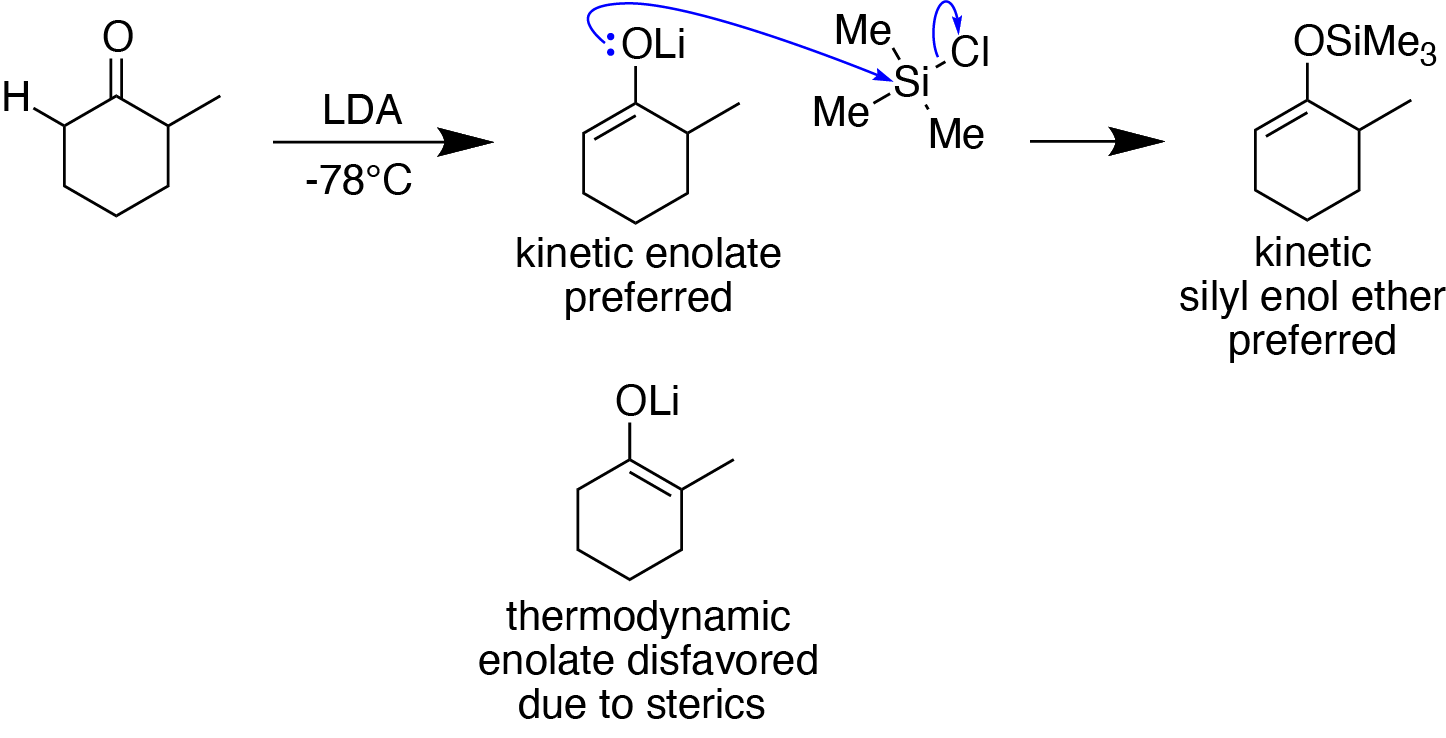
Silyl Enol Ether
In organosilicon chemistry, silyl enol ethers are a class of organic compounds that share the common functional group , composed of an enolate () bonded to a silane () through its oxygen end and an ethene group () as its carbon end. They are important intermediates in organic synthesis. Synthesis Silyl enol ethers are generally prepared by reacting an enolizable carbonyl compound with a silyl electrophile and a base, or just reacting an enolate with a silyl electrophile.Clayden, J., Greeves, N., & Warren, S. (2012). Silyl enol ethers. In ''Organic chemistry'' (Second ed., pp. 466-467). Oxford University Press. Since silyl electrophiles are hard and silicon-oxygen bonds are very strong, the oxygen (of the carbonyl compound or enolate) acts as the nucleophile to form a Si-O single bond. The most commonly used silyl electrophile is trimethylsilyl chloride. To increase the rate of reaction, trimethylsilyl triflate may also be used in the place of trimethylsilyl chloride as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Camphor
Camphor () is a waxy, colorless solid with a strong aroma. It is classified as a terpenoid and a cyclic ketone. It is found in the wood of the camphor laurel (''Cinnamomum camphora''), a large evergreen tree found in East Asia; and in the kapur tree (Dryobalanops, ''Dryobalanops'' sp.), a tall timber tree from South East Asia. It also occurs in some other related trees in the Lauraceae, laurel family, notably ''Ocotea usambarensis''. Rosemary leaves (''Rosmarinus officinalis'') contain 0.05 to 0.5% camphor, while camphorweed (''Heterotheca'') contains some 5%. A major source of camphor in Asia is Ocimum kilimandscharicum, camphor basil (the parent of African blue basil). Camphor can also be synthetically produced from oil of turpentine. The compound is Chirality (chemistry), chiral, existing in two possible enantiomers as shown in the structural diagrams. The structure on the left is the naturally occurring (+)-camphor ((1''R'',4''R'')-bornan-2-one), while its mirror image show ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tms Enol Ether Formation
TMS may refer to: Broadcasting * TMS (entertainment data), data provider * ''Test Match Special'', BBC cricket coverage * ''This Movie Sucks!'', a Canadian TV show on bad movies * '' That Metal Show'', a US TV talk show Media * Hobby of Model Railroading (), a Japanese magazine on railway modelling * Tokyo Motor Show, a biennial auto show in Japan * ''The Morning Show'' (American TV series), an Apple TV drama series Organizations * Telefonia Mobile Sammarinese, a Sammarinese telecommunications company * The Minerals, Metals & Materials Society, a professional organization * Texas Memory Systems, a manufacturer of solid-state drives * TMS Entertainment; formerly Tokyo Movie Shinsha Co., Ltd.; a Japanese animation studio * Toronto Montessori Schools, Richmond Hill, Ontario, Canada * Toyota Motor Sales, U.S.A., Inc. * Tramway Museum Society, the operators of the United Kingdom's National Tramway Museum * Trinity Mathematical Society, UK * TMS (production team), an Englis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Takahashi Taxol Total Synthesis
The Takahashi Taxol total synthesis published by Takashi Takahashi in 2006 is one of several methods in taxol total synthesis.''A Formal Total Synthesis of Taxol Aided by an Automated Synthesizer'' Takayuki Doi, Shinichiro Fuse, Shigeru Miyamoto, Kazuoki Nakai, Daisuke Sasuga and Takashi Takahashi Chemistry an Asian J.; (Article); 2006; 1(3); 370-383. DOAbstract/ref> The method starts from geraniol and differs from the other 6 published methods that it is a formal synthesis (the final product is baccatin III which lacks the amide tail found in taxol itself) and that it is racemic (the product baccatin III is optically inactive). A key feature of the published procedure is that several synthetic steps (construction of rings A, B and C) were performed in an automated synthesizer on a scale up to 300 gram and that purification steps were also automated. A ring synthesis Ring A was synthesised starting from geraniol 1 and involved acylation (acetic anhydride, 4-Dimethylaminopyrid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Glycosylation
A chemical glycosylation reaction involves the coupling of a glycosyl donor, to a glycosyl acceptor forming a glycoside. If both the donor and acceptor are sugars, then the product is an oligosaccharide. The reaction requires activation with a suitable activating reagent. The reactions often result in a mixture of products due to the creation of a new stereogenic centre at the anomeric position of the glycosyl donor. The formation of a glycosidic linkage allows for the synthesis of complex polysaccharides which may play important roles in biological processes and pathogenesis and therefore having synthetic analogs of these molecules allows for further studies with respect to their biological importance. Terminology The glycosylation reaction involves the coupling of a glycosyl donor and a glycosyl acceptor via initiation using an activator under suitable reaction conditions. * A glycosyl donor is a sugar with a suitable leaving group at the anomeric position. This group, u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |