|
Transition Metal Complexes Of Pyridine-N-oxides
Transition metal complexes of pyridine-N-oxides encompass coordination complexes that contain pyridine-N-oxides as ligands. Particularly common are the octahedral homoleptic complexes of the type where M = Mn(II), Fe(II), Co(II), Ni(II). Many variations of pyridine N-oxide are known, such as the dioxides of 2,2'- and 4,4'-Bipyridine, 2,2'-bipyridine. Complexes derived from the trioxide of terpyridine have been crystallized as well. Structure and bonding Pyridine-N-oxides bind to metals through the oxygen. According to X-ray crystallography, the M-O-N angle is approximately 130° in many of these complexes. As reflected by the pKa of 0.79 for , pyridine N-oxides are weakly basic ligands. Their complexes are generally high spin, hence they are kinetically labile. Applications Zinc pyrithione is a coordination compound, coordination complex of a sulfur-substituted pyridine-N-oxide. This zinc complex has useful Fungistatics, fungistatic and bacteriostatic properties.. Referenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CSD CIF PYNONI01
CSD may refer to: Finance * Central securities depository * Confederate States Dollar * Serbian dinar, by previous ISO 4217 code Organizations Education * California School for the Deaf (other), several institutions * Canyons School District, in Utah, US * Cheltenham School District, in Pennsylvania, US * Christina School District, in Delaware, US * Cleveland School District, in Mississippi, US * Cordova School District, in Alaska, US Other organizations * Canteen Stores Department (India), a chain of stores operated by the Indian Ministry of Defence at military bases * CSD Pakistan (Canteen Stores Department), a chain of stores operated by the Pakistani Ministry of Defence * Chartered Society of Designers, a British learned society for various kinds of design work * Commission on Sustainable Development (1992–2013), a former UN agency * Communication Service for the Deaf, an American non-profit company providing ASL services * Congress of Democratic Trade Unions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complexes
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine-N-oxide
Pyridine-''N''-oxide is the heterocyclic compound with the chemical formula, formula C5H5NO. This colourless, hygroscopic solid is the product of the oxidation of pyridine. Its synthesis was first reported by Jakob Meisenheimer, who used peroxybenzoic acid as the oxidant. The compound is used infrequently as an oxidizing reagent in organic synthesis. Structure The structure of pyridine-''N''-oxide is very similar to that of pyridine with respect to the parameters for the ring. The molecule is planar. The N–O distance is 1.34Å. The C–N–C angle is 124°, 7° wider than in pyridine. Synthesis The oxidation of pyridine can be achieved with a number of peroxy acids, including peracetic acid and peroxybenzoic acid. Oxidation can also be effected by a modified Dakin reaction using a urea–hydrogen peroxide complex, and sodium perborate or, using methylrhenium trioxide () as catalyst, with sodium percarbonate. Reactions Pyridine ''N''-oxide is five orders of magnitude less ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis acids and bases, Lewis bases. The nature of metal–ligand bonding can range from covalent bond, covalent to ionic bond, ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acids and bases, Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity (chemistry), reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
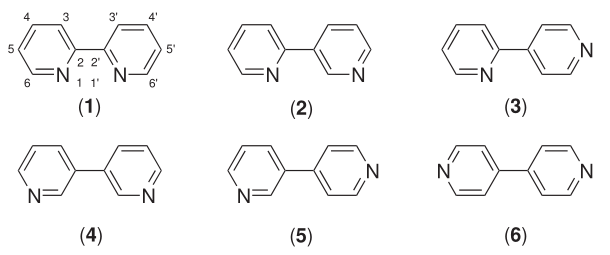
Bipyridine
Bipyridines are a family of organic compounds with the formula (C5H4N)2, consisting of two pyridyl (C5H4N) rings. Pyridine is an aromatic nitrogen-containing heterocycle. The bipyridines are all colourless solids, which are soluble in organic solvents and slightly soluble in water. Bipyridines, especially the 4,4' isomer, are mainly of significance in pesticides. Six isomers of bipyridine exist, but two are prominent. 2,2′-bipyridine, also known as bipyridyl, dipyridyl, and dipyridine, is a popular ligand in coordination chemistry 2,2′-Bipyridine 2,2′-Bipyridine (2,2′-bipy) is a chelating ligand that forms complexes with most transition metal ions that are of broad academic interest. Many of these complexes have distinctive optical properties, and some are of interest for analysis. Its complexes are used in studies of electron and energy transfer, supramolecular, and materials chemistry, and catalysis. 2,2′-Bipyridine is used in the manufacture of diquat. 4, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpyridine
Terpyridine (2,2';6',2"-terpyridine, often abbreviated to Terpy or Tpy) is a heterocyclic compound derived from pyridine. It is a white solid that is soluble in most organic solvents. The compound is mainly used as a ligand in coordination chemistry. Synthesis Terpyridine was first synthesized by G. Morgan and F. H. Burstall in 1932 by the oxidative coupling of pyridines. This method, however, proceeded in low yields. More efficient syntheses have since been described, mainly starting from 2-acetylpyridine. One method produces an enaminone by the reaction of 2-acetylpyridine with N,N-dimethylformamide dimethyl acetal. The base-catalyzed reaction of 2-acetylpyridine with carbon disulfide followed by alkylation with methyl iodide gives C5H4NCOCH=C(SMe)2. Condensation of this species with 2-acetylpyridine forms the related 1,5-diketone, which condenses with ammonium acetate to form a terpyridine. Treatment of this derivative with Raney nickel removes the thioether group. Othe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallography, crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information. X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences between various materials, especially minerals and alloys. The method has also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Pyrithione
Zinc pyrithione (or pyrithione zinc) is a coordination complex of zinc. It has fungistatic (inhibiting the division of fungal cells) and bacteriostatic (inhibiting bacterial cell division) properties and is used in the treatment of seborrhoeic dermatitis and dandruff. Structure of the compound The pyrithione ligands, which are formally monoanions, are chelated to Zn2+ via oxygen and sulfur centers. In the crystalline state, zinc pyrithione exists as a centrosymmetric dimer (see figure), where each zinc is bonded to two sulfur and three oxygen centers. In solution, however, the dimers dissociate via scission of one Zn-O bond. This compound was first described in the 1930s. Pyrithione is the conjugate base derived from 2-mercaptopyridine-''N''-oxide (CAS# 1121-31-9), a derivative of pyridine-''N''-oxide. Uses Medicine Zinc pyrithione can be used to treat dandruff and seborrhoeic dermatitis. It also has antibacterial properties and is effective against many pathoge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Compound
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing chemical compound, compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A Ligand#Polydentate and polyhapto ligand motifs and nomenclature, polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fungistatics
Fungistatics are anti-fungal agents that inhibit the growth of fungus (without killing the fungus). The term ''fungistatic'' may be used as both a noun and an adjective. Fungistatics have applications in agriculture, the food industry, the paint industry, and medicine. Anti-fungal medicines Fluconazole is a fungistatic antifungal medication that is administered orally or intravenously. It is used to treat a variety of fungal infections, especially Candida infections of the vagina ("yeast infections'), mouth, throat, and bloodstream. It is also used to prevent infections in people with weak immune systems, including those with neutropenia due to cancer chemotherapy, transplant patients, and premature babies. Its mechanism of action involves interfering with synthesis of the fungal cell membrane. Itraconazole (R51211), invented in 1984, is a triazole fungistatic antifungal agent prescribed to patients with fungal infections. The drug may be given orally or intravenously. Itraconazol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteriostatic
A bacteriostatic agent or bacteriostat, abbreviated Bstatic, is a biological or chemical agent that stops bacteria from reproducing, while not necessarily killing them otherwise. Depending on their application, bacteriostatic antibiotics, disinfectants, antiseptics and preservatives can be distinguished. When bacteriostatic antimicrobials are used, the duration of therapy must be sufficient to allow host defense mechanisms to eradicate the bacteria. Upon removal of the bacteriostat, the bacteria usually start to grow rapidly. This is in contrast to bactericides, which kill bacteria. Bacteriostats are often used in plastics to prevent growth of bacteria on surfaces. Bacteriostats commonly used in laboratory work include sodium azide (which is acutely toxic) and thiomersal. __TOC__ Bacteriostatic antibiotics Bacteriostatic antibiotics limit the growth of bacteria by interfering with bacterial protein production, DNA replication, or other aspects of bacterial cellular metabolism. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |