|
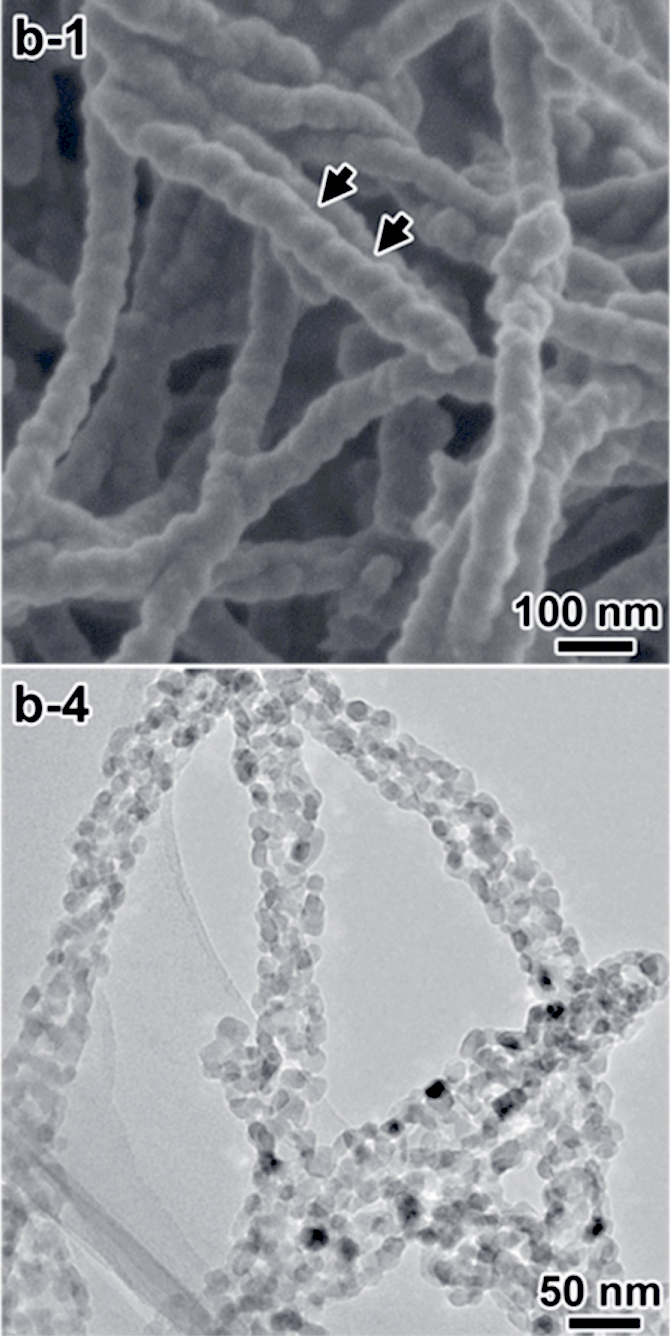
Titanosilicate
A titanosilicate, also called titanium silicate or silicotitanate, is a silicate mineral where some portion of the silicon atoms have been replaced by titanium(IV) atoms. The term most commonly refers to the zeolitic polymorph, also called titanium silicalite, which is a heterogeneous catalyst in industrial chemistry. Applications Titanosilicate catalysts are commonly used in oxidation reactions that employ hydrogen peroxide as an oxidant. The production of propylene oxide by epoxidation of propylene is a major industrial application. Epoxidation by is considered a greener alternative to epoxidation via propylene chlorohydrin, but this reaction of and propylene competes with many side reactions. The formation of titanium coordination complexes in the zeolite structure improves selectivity for the epoxide. Synthesis Titanosilicates can be prepared via crystallisation of silica and titanium dioxide with quaternary ammonium cations as a structure-directing agent. The silic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicalite
Silicalite is an inorganic compound with the formula SiO2. It is one of several forms ( polymorphs) of silicon dioxide. It is a white solid. It consists of tetrahedral silicon centers and two-coordinate oxides. It is prepared by hydrothermal reaction using tetrapropylammonium hydroxide followed by calcining to remove residual ammonium salts. The compound is notable in being ca. 33% porous. It is useful because the material contains (SiO)10 rings that allow sorption of hydrophobic molecules of diameter 0.6 nm. A commercially important modification of silicalite is titanium silicalite. With the formula Si1−xTixO2, it consists of silicalite with Ti doped into some Si sites. Unlike conventional polymorphs of titanium dioxide, the Ti centers in titanium silicalite have tetrahedral coordination geometry. The material is a useful catalyst for the reaction of hydrogen peroxide with propylene to give propylene oxide Propylene oxide is an epoxide with the molecular formula C3H6O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicate Mineral
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, the crystalline forms of silica (silicon dioxide, ) are usually considered to be Silicate mineral#Tectosilicates, tectosilicates, and they are classified as such in the Dana system (75.1). However, the Nickel-Strunz system classifies them as oxide minerals (4.DA). Silica is found in nature as the mineral quartz, and its polymorphism (materials science), polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this carbonate–silicate cycle, geologic cycle. For example, a type of plankton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallisation
Crystallization is a process that leads to solids with highly organized atoms or molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regular organization. Crystallization can occur by various routes including precipitation from solution, freezing of a liquid, or deposition from a gas. Attributes of the resulting crystal can depend largely on factors such as temperature, air pressure, cooling rate, or solute concentration. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles form layers at the crystal's surface and lodge themselves into open inconsistencies such as pores, cracks, etc. Crystallizati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Versalis
Versalis (''Polimeri Europa'' till 5 April 2012) is a wholly owned subsidiary of Italian oil supermajor Eni specializing in the production of chemicals. With more than 7,000 employees and a production of about 5.6 million tons of chemical products in 2023, it is by far the largest chemical company in Italy and one of the largest in Europe. History The history of Versalis dates back to the Enrico Mattei's management era of Eni. In 1953 Mattei obtained full control of Anic, a company specializing in synthetic fuels by coal hydrogenation, and initiated a massive investment program in order to turn it into the chemical and agrichemical arm of Eni. In 1957, following the discovery of substantial natural gas fields off the Adriatic coast, a major fertilizer and petrochemical plant was completed in Ravenna, than in 1960 started producing PVC. Two years later another large plant was set up in Gela, Sicily, close to some offshore oil fields. Other chemical plants were built in Pistic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MCM-41
MCM-41 (Mobil Composition of Matter No. 41) is a mesoporous material with a hierarchical structure from a family of silicate and alumosilicate solids that were first developed by researchers at ExxonMobil, Mobil Oil Corporation and that can be used as catalysts or catalyst supports.Reichinger, M. (2007) ''Poröse Silikate mit hierarchischer Porenstruktur: Synthese von mikro-/mesoporösem MCM-41 und MCM-48 Materialien aus zeolithischen Baueinheiten des MFI-Gerüststrukturtyps'' Dissertation Ruhr-Universität Bochum (in German) Structure MCM-41 consists of a regular arrangement of cyli ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium(IV) Oxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound derived from titanium with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insoluble in water, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at a price of $13.2 billion. Structure In all three of its main dioxides, titanium exhibits octahedral geometry, being bonded to six oxide anions. The oxides in turn are bonded to three Ti centers. The overall crystal structures of rutile and anatase are tetragonal in symmetry whereas brookite is orthorhombic. The oxygen substructur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral
In geometry, an octahedron (: octahedra or octahedrons) is any polyhedron with eight faces. One special case is the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex. Many types of irregular octahedra also exist, including both convex set, convex and non-convex shapes. Combinatorially equivalent to the regular octahedron The following polyhedra are combinatorially equivalent to the regular octahedron. They all have six vertices, eight triangular faces, and twelve edges that correspond one-for-one with the features of it: * Triangular antiprisms: Two faces are equilateral, lie on parallel planes, and have a common axis of symmetry. The other six triangles are isosceles. The regular octahedron is a special case in which the six lateral triangles are also equilateral. * Tetragonal bipyramids, in which at least one of the equatorial quadrilaterals lies on a plane. The regular octahedron is a special case in which all thr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tetrahedron is the simplest of all the ordinary convex polytope, convex polyhedra. The tetrahedron is the three-dimensional case of the more general concept of a Euclidean geometry, Euclidean simplex, and may thus also be called a 3-simplex. The tetrahedron is one kind of pyramid (geometry), pyramid, which is a polyhedron with a flat polygon base and triangular faces connecting the base to a common point. In the case of a tetrahedron, the base is a triangle (any of the four faces can be considered the base), so a tetrahedron is also known as a "triangular pyramid". Like all convex polyhedra, a tetrahedron can be folded from a single sheet of paper. It has two such net (polyhedron), nets. For any tetrahedron there exists a sphere (called th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ZSM-5 Oil Company in 1975, it is widely used in the as a heterogeneous catalyst for reactions.
ZSM-5, Zeolite Socony Mobil–5 (framework type MFI from ZSM-5 (five)), is an aluminosilicate zeolite belonging to the pentasil family of zeolites. Its chemical formula is NanAlnSi96–nO192·16H2O (0 St ...
|
Tetrabutyl Orthotitanate
Titanium butoxide is a metal alkoxide with the formula Ti(OBu)4 ( Bu = –CH2CH2CH2CH3). It is a colorless odorless liquid although aged samples can appear yellowish. Owing to hydrolysis, samples have a weak alcohol-like odor. It is soluble in many organic solvents. Decomposition in water is not hazardous, and therefore titanium butoxide is often used as a liquid source of titanium dioxide, which allows deposition of TiO2 coatings of various shapes and sizes down to the nanoscale. Titanium butoxide is often used to prepare titanium oxide materials and catalysts. Structure and synthesis Like most titanium alkoxides (exception: titanium isopropoxide), Ti(OBu)4 is not a monomer but exists as a cluster (see titanium ethoxide). Nonetheless it is often depicted as a simple monomer. It is produced by treating titanium tetrachloride with butanol Butanol (also called butyl alcohol) is a four-carbon alcohol with a formula of C4 H9 OH, which occurs in five isomeric structures (four s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraethyl Orthosilicate
Tetraethyl orthosilicate, formally named tetraethoxysilane (TEOS), ethyl silicate is the organic chemical compound with the formula Si(OC2H5)4. TEOS is a colorless liquid. It degrades in water. TEOS is the of orthosilicic acid, Si(OH)4. It is the most prevalent alkoxide of silicon. TEOS is a tetrahedral molecule. Like its many analogues, it is prepared by alcoholysis of silicon tetrachloride: :SiCl4 + 4 EtOH → Si(OEt)4 + 4 HCl where Et is the ethyl group, C2H5, and thus EtOH is ethanol. Applications TEOS is mainly used as a crosslinking agent in silicone polymers and as a precursor to silicon dioxide in the semiconductor industry. TEOS is also used as the silica source for synthesis of some zeolites. Other applications include coatings for carpets and other objects. TEOS is used in the production of aerogel. These applications exploit the reactivity of the Si-OR bonds. TEOS has historically been used as an additive to alcohol based rocket fuels to decrease the heat f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |