|
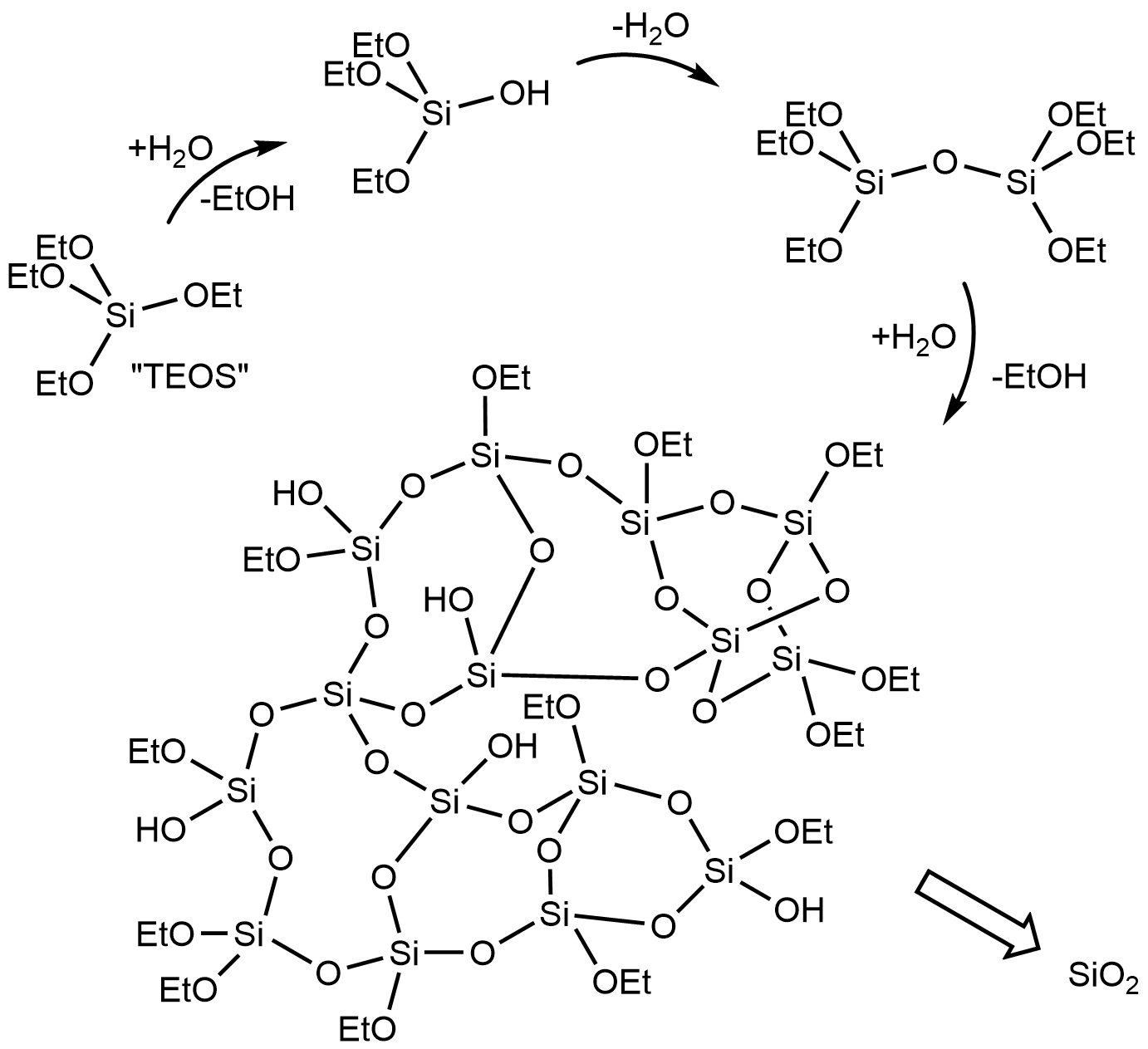
Tetraethyl Orthosilicate
Tetraethyl orthosilicate, formally named tetraethoxysilane (TEOS), ethyl silicate is the organic chemical compound with the formula Si(OC2H5)4. TEOS is a colorless liquid. It degrades in water. TEOS is the of orthosilicic acid, Si(OH)4. It is the most prevalent alkoxide of silicon. TEOS is a tetrahedral molecule. Like its many analogues, it is prepared by alcoholysis of silicon tetrachloride: :SiCl4 + 4 EtOH → Si(OEt)4 + 4 HCl where Et is the ethyl group, C2H5, and thus EtOH is ethanol. Applications TEOS is mainly used as a crosslinking agent in silicone polymers and as a precursor to silicon dioxide in the semiconductor industry. TEOS is also used as the silica source for synthesis of some zeolites. Other applications include coatings for carpets and other objects. TEOS is used in the production of aerogel. These applications exploit the reactivity of the Si-OR bonds. TEOS has historically been used as an additive to alcohol based rocket fuels to decrease the heat f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC Nomenclature Of Inorganic Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Passivation (chemistry)
In physical chemistry and engineering, passivation is coating a material so that it becomes "passive", that is, less readily affected or corroded by the environment. Passivation involves creation of an outer layer of shield material that is applied as a microcoating, created by chemical reaction with the base material, or allowed to build by spontaneous oxidation in the air. As a technique, passivation is the use of a light coat of a protective material, such as metal oxide, to create a shield against corrosion. Passivation of silicon is used during fabrication of microelectronic devices. Undesired passivation of electrodes, called "fouling", increases the circuit resistance so it interferes with some electrochemical applications such as electrocoagulation for wastewater treatment, amperometric chemical sensing, and electrochemical synthesis. When exposed to air, many metals naturally form a hard, relatively inert surface layer, usually an oxide (termed the "native oxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethyl Orthosilicate
Tetramethyl orthosilicate (TMOS) is the chemical compound with the formula Si(OCH3)4. This molecule consists of four methoxy groups bonded to a silicon atom. The basic properties are similar to the more popular tetraethyl orthosilicate, which is usually preferred because the product of hydrolysis, ethanol, is less toxic than methanol. Tetramethyl orthosilicate hydrolyzes to SiO2: :Si(OCH3)4 + 2 H2O → SiO2 + 4 CH3OH In organic synthesis, Si(OCH3)4 has been used to convert ketones and aldehydes to the corresponding ketals and acetal In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments n ...s, respectively.Sakurai, H. "Silicon(IV) Methoxide" in Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons. Safety The hydrolysis of Si(OCH3)4 produces insoluble SiO2 and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pulmonary Edema
Pulmonary edema (British English: oedema), also known as pulmonary congestion, is excessive fluid accumulation in the tissue or air spaces (usually alveoli) of the lungs. This leads to impaired gas exchange, most often leading to shortness of breath ( dyspnea) which can progress to hypoxemia and respiratory failure. Pulmonary edema has multiple causes and is traditionally classified as cardiogenic (caused by the heart) or noncardiogenic (all other types not caused by the heart). Various laboratory tests ( CBC, troponin, BNP, etc.) and imaging studies (chest x-ray, CT scan, ultrasound) are often used to diagnose and classify the cause of pulmonary edema. Treatment is focused on three aspects: * improving respiratory function, * treating the underlying cause, and * preventing further damage and allow full recovery to the lung. Pulmonary edema can cause permanent organ damage, and when sudden (acute), can lead to respiratory failure or cardiac arrest due to hypoxia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs to the ether class of organic compounds. It is a common solvent and was formerly used as a general anesthetic. Production Most diethyl ether is produced as a byproduct of the vapor-phase Hydration reaction, hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid Catalysis, catalysts and can be adjusted to make more ether if the need arises: Vapor-phase Dehydration reaction, dehydration of ethanol over some Aluminium oxide, alumina catalysts can give diethyl ether yields of up to 95%. : Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Uses The dominant use of diethyl ether is as a solvent. One particular application is in the production of cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World Scientific Publishing
World Scientific Publishing is an academic publisher of scientific, technical, and medical books and journals headquartered in Singapore. The company was founded in 1981. It publishes about 600 books annually, with more than 170 journals in various fields. In 1995, World Scientific co-founded the London-based Imperial College Press together with the Imperial College of Science, Technology and Medicine. Company structure The company head office is in Singapore. The Chairman and Editor-in-Chief is Dr Phua Kok Khoo, while the Managing Director is Doreen Liu. The company was co-founded by them in 1981. Imperial College Press In 1995 the company co-founded Imperial College Press, specializing in engineering, medicine and information technology, with Imperial College London Imperial College London, also known as Imperial, is a Public university, public research university in London, England. Its history began with Prince Albert of Saxe-Coburg and Gotha, Prince Albert, h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
John Wiley & Sons
John Wiley & Sons, Inc., commonly known as Wiley (), is an American Multinational corporation, multinational Publishing, publishing company that focuses on academic publishing and instructional materials. The company was founded in 1807 and produces books, Academic journal, journals, and encyclopedias, in print and electronically, as well as online products and services, training materials, and educational materials for undergraduate, graduate, and continuing education students. History The company was established in 1807 when Charles Wiley opened a print shop in Manhattan. The company was the publisher of 19th century American literary figures like James Fenimore Cooper, Washington Irving, Herman Melville, and Edgar Allan Poe, as well as of legal, religious, and other non-fiction titles. The firm took its current name in 1865. Wiley later shifted its focus to scientific, Technology, technical, and engineering subject areas, abandoning its literary interests. Wiley's son Joh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesoporous Silica
Mesoporous silica is a form of silica that is characterised by its mesoporous structure, that is, having pores that range from 2 nm to 50 nm in diameter. According to IUPAC's terminology, mesoporosity sits between microporous (50 nm). Mesoporous silica is a relatively recent development in nanotechnology. The most common types of mesoporous nanoparticles are MCM-41 and Santa Barbara Amorphous-15, SBA-15. Research continues on the particles, which have applications in catalysis, drug delivery and Medical imaging, imaging. Mesoporous ordered silica films have been also obtained with different pore topologies. A compound producing mesoporous silica was patented around 1970. It went almost unnoticed and was reproduced in 1997. Mesoporous silica nanoparticles (MSNs) were independently synthesized in 1990 by researchers in Japan. They were later produced also at Mobil Corporation laboratories and named Mobil Composition of Matter (or Mobil Crystalline Materials, MCM). S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stöber Process
The Stöber process is a chemical process used to prepare silica () particles of controllable and monodisperse, uniform size for applications in materials science. It was pioneering when it was reported by Werner Stöber and his team in 1968, and remains today the most widely used wet chemistry synthetic approach to silica nanoparticles. It is an example of a sol-gel process wherein a molecular precursor (chemistry), precursor (typically tetraethylorthosilicate) is first hydrolysis, reacted with water in an alcoholic solution, the resulting molecules then condensation, joining together to build larger structures. The reaction produces silica particles with diameters ranging from 50 to 2000 nanometre, nm, depending on conditions. The process has been actively researched since its discovery, including efforts to understand its chemical kinetics, kinetics and reaction mechanism, mechanisma particle aggregation model was found to be a better fit for the experimental data than the i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. The rate increase occurs because the catalyst allows the reaction to occur by an alternative mechanism which may be much faster than the noncatalyzed mechanism. However the noncatalyzed mechanism does remain possible, so that the total rate (catalyzed plus noncatalyzed) can only increase in the presence of the catalyst and never decrease. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acid–base theory, Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Acid–base reaction#Arrhenius theory, Arrhenius acids. Johannes Nicolaus Brønsted, Brønsted and Martin Lowry, Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |