|
Thioacetic Acid
Thioacetic acid is an organosulfur compound with the molecular formula . It is a thioic acid: the sulfur Structural analog, analogue of acetic acid (), as implied by the ''thio-'' prefix. It is a yellow liquid with a strong thiol-like odor. It is used in organic synthesis for the introduction of thiol groups () in molecules. Synthesis and properties Thioacetic acid is prepared by the reaction of acetic anhydride with hydrogen sulfide: : It has also been produced by the action of phosphorus pentasulfide on glacial acetic acid, followed by distillation. : Thioacetic acid is typically contaminated by acetic acid. The compound exists exclusively as the thiol tautomer, consistent with the strength of the double bond. Reflecting the influence of hydrogen-bonding, the boiling point (93 °C) and melting points are 20 and 75 K lower than those for acetic acid. Reactivity Acidity With a p''K''a near 3.4, thioacetic acid is about 15 times more acidic than acetic acid. The conju ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Thioacetate
Potassium thioacetate is an organosulfur compound and a salt with the formula . This white, water-soluble solid is used as a reagent for preparing thioacetate esters and other derivatives. Synthesis and reactions Potassium thioacetate, which is commercially available, can be prepared by combining acetyl chloride and potassium hydrogen sulfide: : It arises also by the neutralization of thioacetic acid with potassium hydroxide. Use in preparation of thiols In a common application, potassium thioacetate is combined with alkylating agents to give thioacetate esters (X = halide): : Hydrolysis of these esters affords thiol In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...s: : The thioacetate esters can also be cleaved with methanethiol in the presence of stoichiometric base, as ill ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-nitrophenol
4-Nitrophenol (also called ''p''-nitrophenol or 4-hydroxynitrobenzene) is a phenolic compound that has a nitro group at the opposite position of the hydroxyl group on the benzene ring. Properties 4-nitro phenol is a slightly yellow, crystalline material, moderately toxic. It shows two polymorphs in the crystalline state. The alpha-form is colorless pillars, unstable at room temperature, and stable toward sunlight. The beta-form is yellow pillars, stable at room temperature, and gradually turns red upon irradiation of sunlight. Usually 4-nitrophenol exists as a mixture of these two forms. In solution, 4-nitrophenol has a dissociation constant (pKa) of 7.15 at 25 °C. Preparation From phenol 4-Nitrophenol can be prepared by nitration of phenol using dilute nitric acid at room temperature. The reaction produces a mixture of 2-nitrophenol and 4-nitrophenol. : Uses pH indicator 4-Nitrophenol can be used as a pH indicator. A solution of 4-nitrophenol appears colorle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paracetamol
Paracetamol, or acetaminophen, is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely available over-the-counter drug sold under various brand names, including Tylenol and Panadol. Paracetamol relieves pain in both acute mild migraine and episodic tension headache. At a standard dose, paracetamol slightly reduces fever, though it is inferior to ibuprofen in that respect and the benefits of its use for fever are unclear, particularly in the context of fever of viral origins. The aspirin/paracetamol/caffeine combination also helps with both conditions when the pain is mild and is recommended as a Therapy#Lines of therapy, first-line treatment for them. Paracetamol is effective for post-surgical pain, but is inferior to ibuprofen for this purpose. The paracetamol/ibuprofen combination increases the drugs' potency and is superior to either drug alone. The pain relief paracetamol provides in osteoarthritis is small and clinica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Thioacetate
Potassium thioacetate is an organosulfur compound and a salt with the formula . This white, water-soluble solid is used as a reagent for preparing thioacetate esters and other derivatives. Synthesis and reactions Potassium thioacetate, which is commercially available, can be prepared by combining acetyl chloride and potassium hydrogen sulfide: : It arises also by the neutralization of thioacetic acid with potassium hydroxide. Use in preparation of thiols In a common application, potassium thioacetate is combined with alkylating agents to give thioacetate esters (X = halide): : Hydrolysis of these esters affords thiol In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...s: : The thioacetate esters can also be cleaved with methanethiol in the presence of stoichiometric base, as ill ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arkivoc
''Arkivoc'' (''Archive for Organic Chemistry'') is a peer-reviewed open access scientific journal covering all aspects of organic chemistry. It is published by the non-profit organization Arkat USA, which was established in 2000 through a personal donation from Alan R. Katritzky and Linde Katritzky. ''Arkivoc'' is the primary publication of Arkat USA. According to the ''Journal Citation Reports'', the journal has a 2014 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a type of journal ranking. Journals with higher impact factor values are considered more prestigious or important within their field. The Impact Factor of a journa ... of 1.165, ranking it 37th out of 57 journals in the category "Chemistry, Organic". Abstracting and Indexing According to the Journal Citation Reports, the journal has a 2018 impact factor of 1.253. The journal is indexed in Web of Science: Science Citation Index Expanded. References External lin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
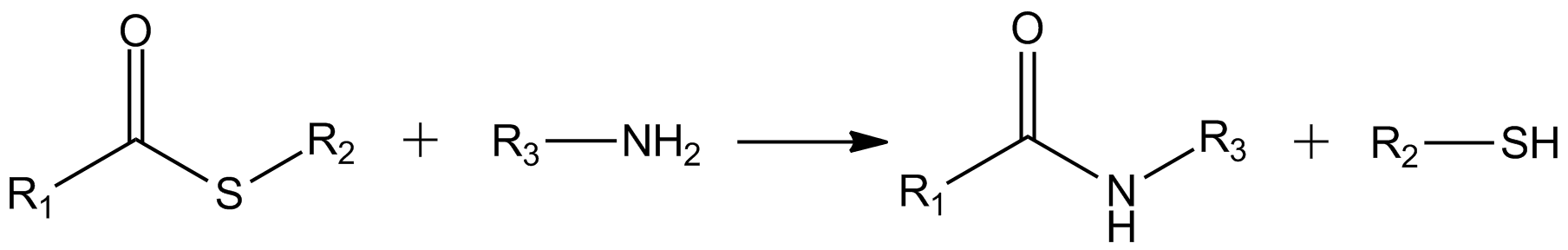
Thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid () with a thiol (). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. The R and R' represent organyl groups, or H in the case of R. Synthesis One route to thioesters involves the reaction of an acid chloride with an alkali metal salt of a thiol: : Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation of potassium thioacetate: : Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exocyclic
In organic chemistry, an alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. Cycloalkanes The simplest alicyclic compounds are the monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cycloheptane, cyclooctane, and so on. Bicyclic alkanes include decalin, housane, and norbornane. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. The mode of ring-closing in the formation of many alicyclic compounds can be predicted by Baldwin's rules. Otto Wallach, a German chemist, received the 1910 Nobel Prize in Chemistry for his work on alicyclic compounds. Cycloalkenes Monocyclic cycloalkenes are cyclopropene, cyclobutene, cyclopentene, cyclohexene, cycloheptene, cyclooctene, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions differ from electrophilic additions in that the former reactions involve the group to which atoms are added accepting electron pairs, whereas the latter reactions involve the group donating electron pairs. Addition to carbon–heteroatom double bonds Nucleophilic addition reactions of nucleophiles with electrophilic double or triple bond (π bonds) create a new carbon center with two additional single, or σ, bonds.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Addition of a nucleophile to carbon–heteroatom double or triple bonds such as >C=O or -C≡N show great variety. These types of bonds are polar (have a large difference in electronegativit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing. Ageing Biogerontology Biological processes Causes of death Cellular processes Gerontology Life extension Metabolic disorders Metabolism Old age Time in life {{CatAutoTOC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |