|
4-Nitrophenol
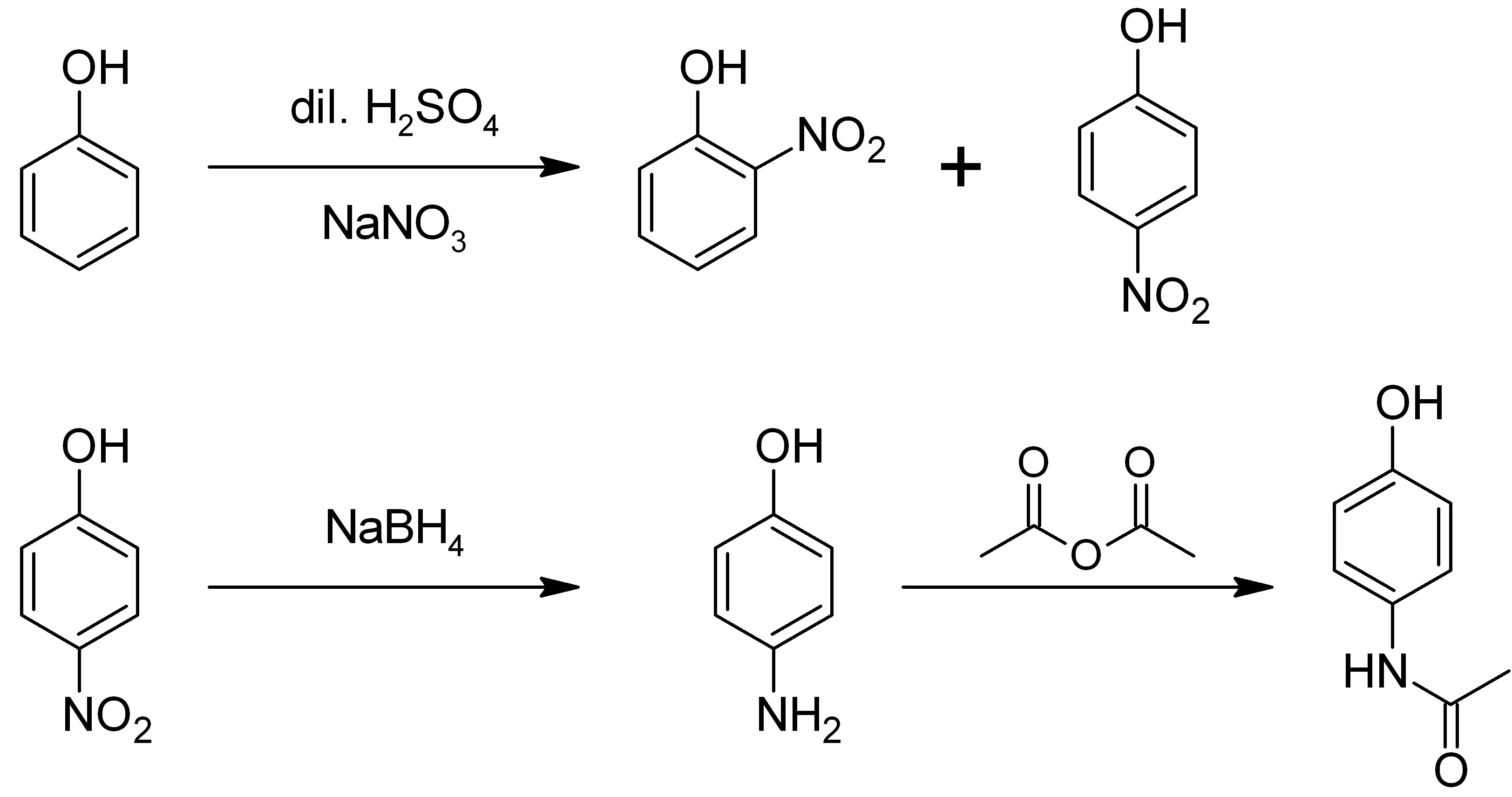
4-Nitrophenol (also called ''p''-nitrophenol or 4-hydroxynitrobenzene) is a phenolic compound that has a nitro group at the opposite position of the hydroxyl group on the benzene ring. Properties 4-nitro phenol is a slightly yellow, crystalline material, moderately toxic. It shows two polymorphs in the crystalline state. The alpha-form is colorless pillars, unstable at room temperature, and stable toward sunlight. The beta-form is yellow pillars, stable at room temperature, and gradually turns red upon irradiation of sunlight. Usually 4-nitrophenol exists as a mixture of these two forms. In solution, 4-nitrophenol has a dissociation constant (pKa) of 7.15 at 25 °C. Preparation From phenol 4-Nitrophenol can be prepared by nitration of phenol using dilute nitric acid at room temperature. The reaction produces a mixture of 2-nitrophenol and 4-nitrophenol. : Uses pH indicator 4-Nitrophenol can be used as a pH indicator. A solution of 4-nitrophenol appears colorle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrophenols
Nitrophenols are compounds of the formula HOC6H5−x(NO2)x. The conjugate bases are called nitrophenolates. Nitrophenols are more acidic than phenol itself. Mono-nitrophenols with the formula HOC6H4NO2. Three isomeric nitrophenols exist: * ''o''-Nitrophenol (2-nitrophenol; OH and NO2 groups are neighboring, a yellow solid. * ''m''-Nitrophenol (3-nitrophenol, CAS number: 554-84-7), a yellow solid (m.p. 97 °C) and precursor to the drug mesalazine (5-aminosalicylic acid). It can be prepared by nitration of aniline followed by replacement of the amino group via its diazonium derivative. * ''p''-Nitrophenol, yellow solid is a precursor to the rice herbicide fluorodifen, the pesticide parathion, and the human analgesic paracetamol (also known as acetaminophen). The mononitrated phenols are often hydrogenated to the corresponding aminophenols that are also useful industrially. Di- and trinitrophenols * 2,4-Dinitrophenol (m.p. 83 °C) is a moderately strong acid (pKa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-nitrophenol
Nitrophenols are compounds of the formula HOC6H5−x(NO2)x. The conjugate bases are called nitrophenolates. Nitrophenols are more acidic than phenol itself. Mono-nitrophenols with the chemical formula, formula HOC6H4NO2. Three isomeric nitrophenols exist: * ''o''-Nitrophenol (2-nitrophenol; OH and NO2 groups are neighboring, a yellow solid. * ''m''-Nitrophenol (3-nitrophenol, CAS number: 554-84-7), a yellow solid (m.p. 97 °C) and precursor to the drug mesalazine (5-aminosalicylic acid). It can be prepared by nitration of aniline followed by replacement of the amino group via its diazonium derivative. * 4-Nitrophenol, ''p''-Nitrophenol, yellow solid is a precursor to the rice herbicide fluorodifen, the pesticide parathion, and the human analgesic paracetamol (also known as acetaminophen). The mononitrated phenols are often hydrogenation, hydrogenated to the corresponding aminophenols that are also useful industrially. Di- and trinitrophenols * 2,4-Dinitrophenol (m.p. 8 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-aminophenol
4-Aminophenol (or ''para''-aminophenol or ''p''-aminophenol) is an organic compound with the formula H2NC6H4OH. Typically available as a white powder, it is commonly used as a developer for black-and-white film, marketed under the name Rodinal. Reflecting its slightly hydrophilic character, the white powder is moderately soluble in alcohols and can be recrystallized from hot water. In the presence of a base, it oxidizes readily. The methylated derivatives ''N''-methylaminophenol and ''N'',''N''-dimethylaminophenol are of commercial value. The compound is one of three isomeric aminophenols, the other two being 2-aminophenol and 3-aminophenol. __TOC__ Preparation From phenol It is produced from phenol by nitration followed by reduction with iron. Alternatively, the partial hydrogenation of nitrobenzene affords phenylhydroxylamine, which rearranges primarily to 4-aminophenol ( Bamberger rearrangement). :C6H5NO2 + 2 H2 → C6H5NHOH + H2O :C6H5NHOH → HOC6H4NH2 From ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paracetamol
Paracetamol, or acetaminophen, is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely available over-the-counter drug sold under various brand names, including Tylenol and Panadol. Paracetamol relieves pain in both acute mild migraine and episodic tension headache. At a standard dose, paracetamol slightly reduces fever, though it is inferior to ibuprofen in that respect and the benefits of its use for fever are unclear, particularly in the context of fever of viral origins. The aspirin/paracetamol/caffeine combination also helps with both conditions when the pain is mild and is recommended as a Therapy#Lines of therapy, first-line treatment for them. Paracetamol is effective for post-surgical pain, but is inferior to ibuprofen for this purpose. The paracetamol/ibuprofen combination increases the drugs' potency and is superior to either drug alone. The pain relief paracetamol provides in osteoarthritis is small and clinica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methemoglobinemia
Methemoglobinemia, or methaemoglobinaemia, is a condition of elevated methemoglobin in the blood. Symptoms may include headache, dizziness, shortness of breath, nausea, poor muscle coordination, and blue-colored skin (cyanosis). Complications may include seizures and heart arrhythmias. Methemoglobinemia can be due to certain medications, chemicals, or food or it can be inherited. Substances involved may include benzocaine, nitrites, or dapsone. The underlying mechanism involves some of the iron in hemoglobin being converted from the ferrous [Fe2+] to the ferric [Fe3+] form. The diagnosis is often suspected based on symptoms and a Hypoxia (medical), low blood oxygen that does not improve with oxygen therapy. Diagnosis is confirmed by a blood gas. Treatment is generally with oxygen therapy and methylene blue. Other treatments may include vitamin C, exchange transfusion, and hyperbaric oxygen therapy. Outcomes are generally good with treatment. Methemoglobinemia is relatively unc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methaemoglobin
Methemoglobin (British: methaemoglobin, shortened MetHb) (pronounced "met-hemoglobin") is a hemoglobin ''in the form of metalloprotein'', in which the iron in the heme group is in the Fe3+ (ferric) state, not the Fe2+ (ferrous) of normal hemoglobin. Sometimes, it is also referred to as ferrihemoglobin. Methemoglobin cannot bind oxygen, which means it cannot carry oxygen to tissues. It is bluish chocolate-brown in color. In human blood a trace amount of methemoglobin is normally produced spontaneously, but when present in excess the blood becomes abnormally dark bluish brown. The NADH-dependent enzyme methemoglobin reductase (diaphorase, a type of diaphorase) is responsible for converting methemoglobin back to hemoglobin. Normally one to two percent of a person's hemoglobin is methemoglobin; a higher percentage than this can be genetic or caused by exposure to various chemicals and depending on the level can cause health problems known as methemoglobinemia. A higher level of methemog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly corrosive base (chemistry), base and alkali that decomposes lipids and proteins at ambient temperatures and at high concentrations may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the making of wood pulp and paper, tex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include Ribozyme, catalytic RNA molecules, also called ribozymes. They are sometimes descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosidase
In biochemistry, glycoside hydrolases (also called glycosidases or glycosyl hydrolases) are a class of enzymes which catalyze the hydrolysis of glycosidic bonds in complex sugars. They are extremely common enzymes, with roles in nature including degradation of biomass such as cellulose (cellulase), hemicellulose, and starch (amylase), in anti-bacterial defense strategies (e.g., lysozyme), in pathogenesis mechanisms (e.g., viral neuraminidases) and in normal cellular function (e.g., trimming mannosidases involved in ''N''-linked glycoprotein biosynthesis). Together with glycosyltransferases, glycosidases form the major catalytic machinery for the synthesis and breakage of glycosidic bonds. Occurrence and importance Glycoside hydrolases are found in essentially all domains of life. In prokaryotes, they are found both as intracellular and extracellular enzymes that are largely involved in nutrient acquisition. One of the important occurrences of glycoside hydrolases in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioaccumulation
Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance faster than it can be lost or eliminated by catabolism and excretion. Thus, the longer the biological half-life of a toxic substance, the greater the risk of chronic poisoning, even if environmental levels of the toxin are not very high. Bioaccumulation, for example in fish, can be predicted by models. Hypothesis for molecular size cutoff criteria for use as bioaccumulation potential indicators are not supported by data. Biotransformation can strongly modify bioaccumulation of chemicals in an organism. Toxicity induced by metals is associated with bioaccumulation and biomagnification. Storage or uptake of a metal faster than it is metabolized and excreted leads to the accumulation of that metal. The presence of various chemicals and harmful substances in the environment can be analyzed and assessed with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonic Anhydrase
The carbonic anhydrases (or carbonate dehydratases) () form a family of enzymes that catalyst, catalyze the interconversion between carbon dioxide and water and the Dissociation (chemistry), dissociated ions of carbonic acid (i.e. bicarbonate and hydrogen ions). The active site of most carbonic anhydrases contains a zinc ion. They are therefore classified as metalloprotein, metalloenzymes. The enzyme maintains Acid–base homeostasis, acid-base balance and helps transport carbon dioxide. Carbonic anhydrase helps maintain acid–base homeostasis, regulate pH, and fluid balance. Depending on its location, the role of the enzyme changes slightly. For example, carbonic anhydrase produces acid in the stomach lining. In the kidney, the control of bicarbonate ions influences the water content of the cell. The control of bicarbonate ions also influences the water content in the eyes. Inhibitors of carbonic anhydrase are used to treat glaucoma, the excessive build-up of water in the eyes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |