Thioester on:
[Wikipedia]
[Google]
[Amazon]
In
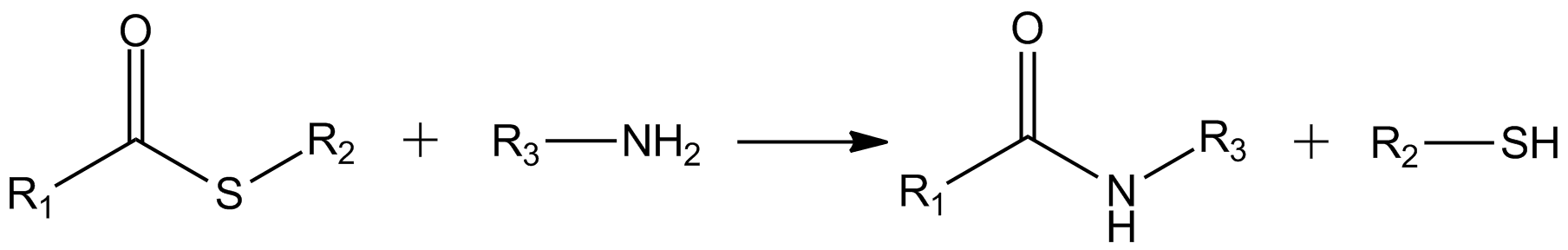 This reaction is exploited in native chemical ligation, a protocol for peptide synthesis.
In a related reaction, thioesters can be converted into esters. Thioacetate esters can also be cleaved with methanethiol in the presence of stoichiometric base, as illustrated in the preparation of pent-4-yne-1-thiol:
:
:
A reaction unique to thioesters is the Fukuyama coupling, in which the thioester is coupled with an organozinc halide by a palladium catalyst to give a ketone.
:
This reaction is exploited in native chemical ligation, a protocol for peptide synthesis.
In a related reaction, thioesters can be converted into esters. Thioacetate esters can also be cleaved with methanethiol in the presence of stoichiometric base, as illustrated in the preparation of pent-4-yne-1-thiol:
:
:
A reaction unique to thioesters is the Fukuyama coupling, in which the thioester is coupled with an organozinc halide by a palladium catalyst to give a ketone.
:
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
() with a thiol (). In biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
, the best-known thioesters are derivatives of coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the Fatty acid metabolism#Synthesis, synthesis and Fatty acid metabolism#.CE.B2-Oxidation, oxidation of fatty acids, and the oxidation of pyruvic acid, pyruvate in the citric ac ...
, e.g., acetyl-CoA.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. The R and R' represent organyl groups, or H in the case of R.
Synthesis
One route to thioesters involves the reaction of an acid chloride with an alkali metal salt of a thiol: : Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared byalkylation Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
of potassium thioacetate:
:
The analogous alkylation of an acetate salt is rarely practiced. The alkylation can be conducted using Mannich bases and the thiocarboxylic acid:
:
Thioesters can be prepared by condensation of thiols and carboxylic acids in the presence of dehydrating agents:
:
A typical dehydration agent is DCC. Efforts to improve the sustainability of thioester synthesis have also been reported utilising safer coupling reagent T3P and greener solvent cyclopentanone. Acid anhydrides and some lactones also give thioesters upon treatment with thiols in the presence of a base.
Thioesters can be conveniently prepared from alcohols by the Mitsunobu reaction, using thioacetic acid.
They also arise via carbonylation of alkynes and alkenes in the presence of thiols.
Reactions
Thioesters hydrolyze to thiols and the carboxylic acid: : The carbonyl center in thioesters is more reactive toward amine than oxygen nucleophiles, giving amides: : This reaction is exploited in native chemical ligation, a protocol for peptide synthesis.
In a related reaction, thioesters can be converted into esters. Thioacetate esters can also be cleaved with methanethiol in the presence of stoichiometric base, as illustrated in the preparation of pent-4-yne-1-thiol:
:
:
A reaction unique to thioesters is the Fukuyama coupling, in which the thioester is coupled with an organozinc halide by a palladium catalyst to give a ketone.
:
This reaction is exploited in native chemical ligation, a protocol for peptide synthesis.
In a related reaction, thioesters can be converted into esters. Thioacetate esters can also be cleaved with methanethiol in the presence of stoichiometric base, as illustrated in the preparation of pent-4-yne-1-thiol:
:
:
A reaction unique to thioesters is the Fukuyama coupling, in which the thioester is coupled with an organozinc halide by a palladium catalyst to give a ketone.
:
Biochemistry
Thioesters are common intermediates in many biosynthetic reactions, including the formation and degradation offatty acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...
s and mevalonate, precursor to steroids. Examples include malonyl-CoA, acetoacetyl-CoA, propionyl-CoA, cinnamoyl-CoA, and acyl carrier protein (ACP) thioesters. Acetogenesis proceeds via the formation of acetyl-CoA. The biosynthesis of lignin, which comprises a large fraction of the Earth's land biomass, proceeds via a thioester derivative of caffeic acid. These thioesters arise analogously to those prepared synthetically, the difference being that the dehydration agent is ATP. In addition, thioesters play an important role in the tagging of proteins with ubiquitin, which tags the protein for degradation.
Oxidation of the sulfur atom in thioesters ( thiolactones) is postulated in the bioactivation of the antithrombotic prodrugs ticlopidine, clopidogrel, and prasugrel.
Thioesters and the origin of life
As posited in a "Thioester World", thioesters are possible precursors to life. As Christian de Duve explains:It is revealing that thioesters are obligatory intermediates in several key processes in which ATP is either used or regenerated. Thioesters are involved in the synthesis of allHowever, due to the high free energy change of thioester's hydrolysis and correspondingly their low equilibrium constants, it is unlikely that these compounds could have accumulated abiotically to any significant extent especially in hydrothermal vent conditions.esters In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ..., including those found in complexlipid Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing ...s. They also participate in the synthesis of a number of other cellular components, including peptides,fatty acid In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...s,sterol A sterol is any organic compound with a Skeletal formula, skeleton closely related to Cholestanol, cholestan-3-ol. The simplest sterol is gonan-3-ol, which has a formula of , and is derived from that of gonane by replacement of a hydrogen atom on ...s,terpene Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predomi ...s, porphyrins, and others. In addition, thioesters are formed as key intermediates in several particularly ancient processes that result in the assembly of ATP. In both these instances, the thioester is closer than ATP to the process that uses or yields energy. In other words, thioesters could have actually played the role of ATP in a "thioester world" initially devoid of ATP. Eventually, hesethioesters could have served to usher in ATP through its ability to support the formation of bonds betweenphosphate group Phosphates are the naturally occurring form of the element phosphorus. In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosp ...s.
Thionoesters
Thionoesters are isomeric with thioesters. In a thionoester, sulfur replaces the carbonyl oxygen in an ester. Methyl thionobenzoate is C6H5C(S)OCH3. Such compounds are typically prepared by the reaction of the thioacyl chloride with an alcohol. They can also be made by the reaction of Lawesson's reagent with esters or by treating pinner salts with hydrogen sulfide. Various thionoesters may be prepared through the transesterification of an existing methyl thionoester with an alcohol under base-catalyzed conditions. Xanthates andthioamide
A thioamide (rarely, thionamide, but also known as thiourylenes) is a functional group with the general structure , where are any groups (typically organyl groups or hydrogen). Analogous to amides, thioamides exhibit greater multiple bond charact ...
s can be transformed to thionoesters under metal-catalyzed cross-coupling conditions.
See also
* Thiocarboxylic acid * Thiocarbonate * Liebeskind–Srogl coupling * Aldrithiol-2References
{{Functional group Functional groups Origin of life