|
Tetraboric Acid
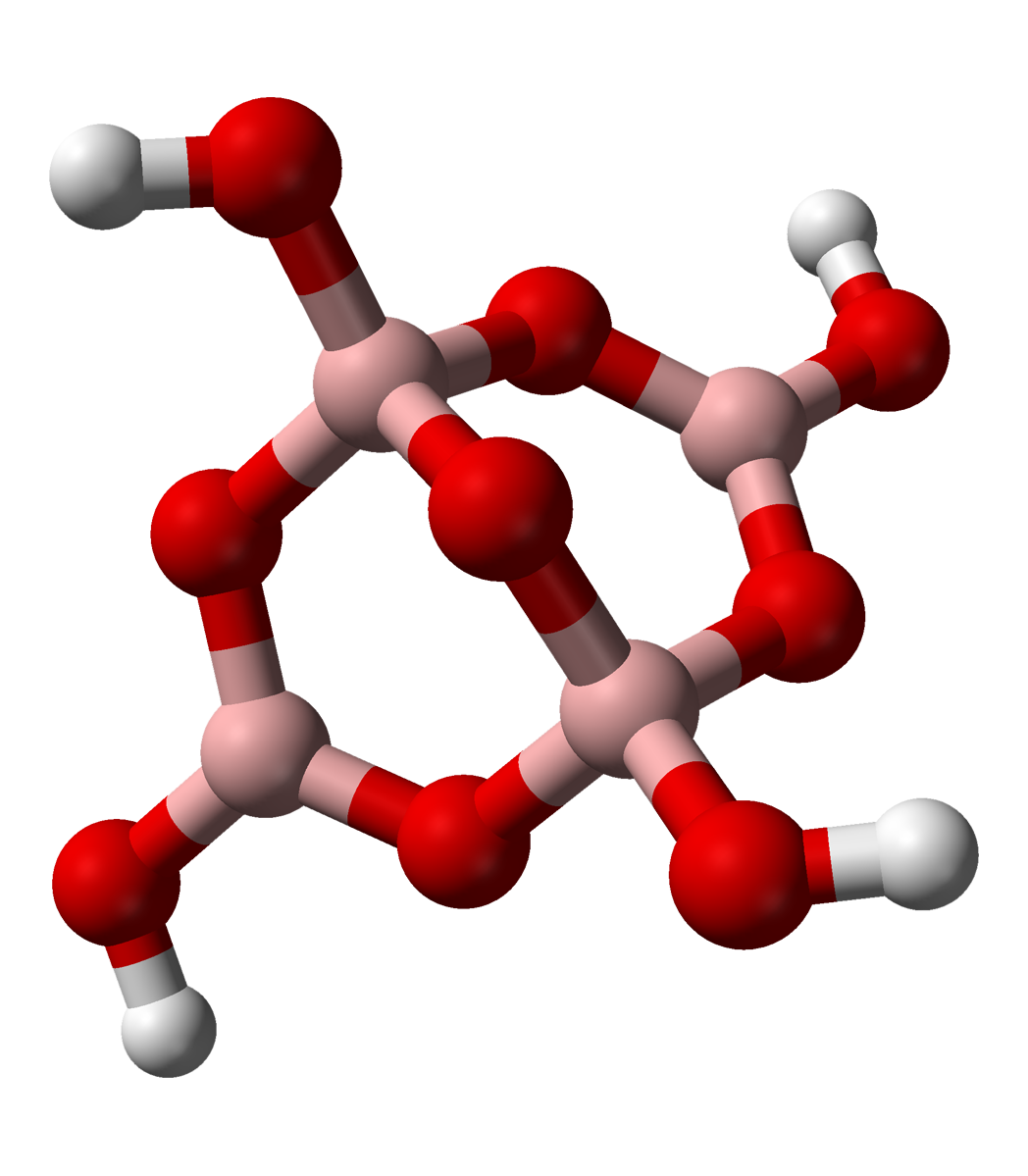
Tetraboric acid or pyroboric acid is a chemical compound with empirical formula . It is a colourless water-soluble solid formed by the dehydration or polymerization of boric acid. Tetraboric acid is formally the parent acid of the tetraborate anion . Preparation Tetraboric acid can be obtained by heating orthoboric acid Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolv ... above about 170 °C: : 4 → + 5 References Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Abdullah Selim Parlakyigit and Celaletdin Ergun (2022): "Facile synthesis method for in situ composites of TiB2/B4C and ZrB2/B4C." ''Journal of the Australian Ceramic Society'', volume 58, pages 411–420. Gurwinder Kaur, Shagun Kainth, Rohit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraborate In chemistry, tetraborate or pyroborate is an anion (negative ion) with formula ; or a salt containing that anion, such as sodium tetraborate, . It is one of the boron oxoacids, that is, a borate. The name is also applied to the hydrated ion as present in borax The ion occurs in boric acid solutions at neutral pH, being formed by condensation of orthoborate and tetrahydroxyborate anions: : 2 B(OH)3 + 2 ⇌ + 5 H2O The tetraborate anion (tetramer) includes two tetrahedral and two trigonal boron atoms symmetrically assembled in a fused bicyclic structure. The two tetrahedral boron atoms are linked together by a common oxygen atom, and each also bears a negative net charge brought |