Tetraborate on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 In
In chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, tetraborate or pyroborate is an anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
(negative ion) with formula ; or a salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
containing that anion, such as sodium tetraborate
The BORAX Experiments were a series of safety experiments on boiling water reactor, boiling water nuclear reactors conducted by Argonne National Laboratory in the 1950s and 1960s at the National Reactor Testing Station in eastern Idaho.
, . It is one of the boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
oxoacids, that is, a borate
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt of such anions, such as sodium metaborate, and borax . The name also refers to esters of su ...
.
The name is also applied to the hydrated ion as present in borax
The BORAX Experiments were a series of safety experiments on boiling water nuclear reactors conducted by Argonne National Laboratory in the 1950s and 1960s at the National Reactor Testing Station in eastern Idaho.
The ion occurs in boric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white ...
solutions at neutral pH, being formed by condensation of orthoborate and tetrahydroxyborate anions:
: 2 B(OH)3 + 2 ⇌ + 5 H2O
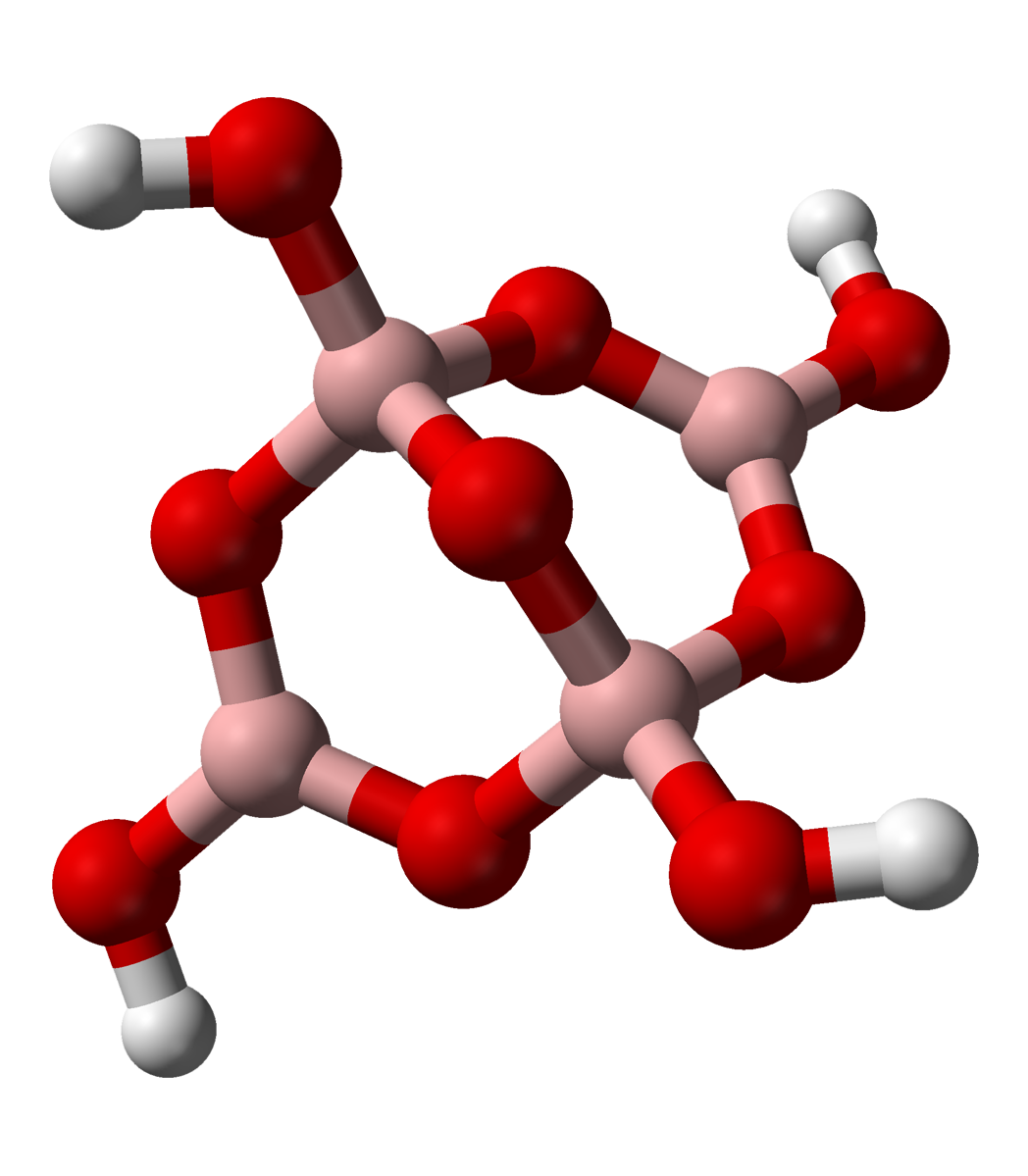
The tetraborate anion (tetramer
A tetramer () (''tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula ...
) includes two tetrahedral and two trigonal boron atoms symmetrically assembled in a fused bicyclic structure. The two tetrahedral boron atoms are linked together by a common oxygen atom, and each also bears a negative net charge brought by the supplementary OH− groups laterally attached to them. This intricate molecular anion also exhibits three rings: two fused distorted hexagonal (boroxole) rings and one distorted octagonal ring. Each ring is made of a succession of alternate boron and oxygen atoms. Boroxole rings are a very common structural motif in polyborate ions.
The hydrated tetraborate anion occurs in the mineral borax
The BORAX Experiments were a series of safety experiments on boiling water nuclear reactors conducted by Argonne National Laboratory in the 1950s and 1960s at the National Reactor Testing Station in eastern Idaho.
(sodium tetraborate octahydrate) with the formula Na2 4O5(OH)4�8H2O. The borax chemical formula is also commonly written in a more compact notation as Na2B4O7·10H2O. Sodium borate can be obtained in high purity and so can be used to make a standard solution
In analytical chemistry, a standard solution (titrant or titrator) is a solution containing an accurately known concentration. Standard solutions are generally prepared by dissolving a solute of known mass into a solvent to a precise volume, or by ...
in titrimetric analysis..
References
Boron oxyanions Borates Heterocyclic compounds with 2 rings Bridged heterocyclic compounds Boron heterocycles Oxygen heterocycles {{salt-stub