|
Tellurophene
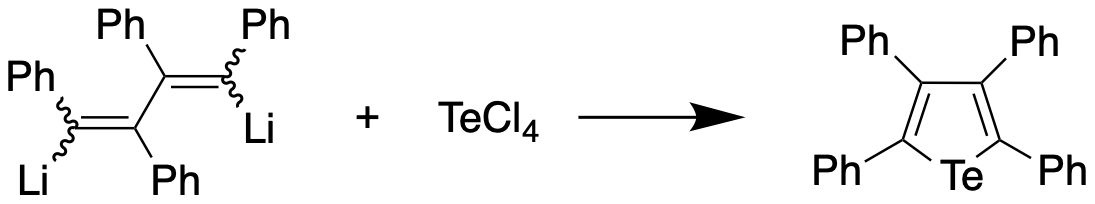
Tellurophenes are the tellurium analogue of thiophenes and selenophenes. Synthesis The first preparation of a tellurophene, tetraphenyltellurophene, was reported in 1961 by Braye et al. by reacting 1,4-dilithiotetraphenylbutadiene with tellurium tetrachloride, with the former synthesized by reaction of diphenylacetylene and lithium metal. The tellurophene, upon recrystallization from a dichloromethane/ethanol mixture, was obtained in 56% yield, and found to appear as yellow-orange crystals with a melting point of 239-239.5 °C. The same compound was obtained from 1,4-diiodotetraphenylbutadiene and lithium telluride in 82% yield. In 1966, Mack report a synthesis of an unsubstituted tellurophene through the reaction of sodium telluride with diacetylene in methanol at 20 °C. This method could be generalised to prepare 2,5-derivatives of tellurophene by selecting a suitably-substituted diacetylene precursor. The product was obtained as a pale yellow liquid with a melti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schlenk Line
The Schlenk line (also vacuum gas manifold) is a commonly used chemistry apparatus developed by Wilhelm Schlenk. It consists of a dual manifold with several ports. One manifold is connected to a source of purified inert gas, while the other is connected to a vacuum pump. The inert-gas line is vented through an oil bubbler, while solvent vapors and gaseous reaction products are prevented from contaminating the vacuum pump by a liquid-nitrogen or dry-ice/ acetone cold trap. Special stopcocks or Teflon taps allow vacuum or inert gas to be selected without the need for placing the sample on a separate line. Schlenk lines are useful for safely and successfully manipulating moisture- and air-sensitive compounds. The vacuum is also often used to remove the last traces of solvent from a sample. Vacuum and gas manifolds often have many ports and lines, and with care, it is possible for several reactions or operations to be run simultaneously. When the reagents are highly suscepti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enyne
In organic chemistry, an enyne is an organic compound containing a double bond (alkene) and a triple bond (alkyne). It is called a conjugated enyne when the double and triple bonds are conjugated. The term is a contraction of the terms alkene and alkyne. The simplest enyne is vinylacetylene. See also * Enyne metathesis *Enediyne *Polyyne In organic chemistry, a polyyne () is any organic compound with alternating single and triple bonds; that is, a series of consecutive alkynes, with ''n'' greater than 1. These compounds are also called polyacetylenes, especially in the natural ... References Chemical nomenclature Alkene derivatives Alkyne derivatives Conjugated hydrocarbons {{organic-chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The Chemical Society, Perkin Transactions 2
Perkin Transactions is a scientific journal In academic publishing, a scientific journal is a periodical publication intended to further the progress of science, usually by reporting new research. Content Articles in scientific journals are mostly written by active scientists such as s ... devoted to organic chemistry published from 1997 to 2002 by the Royal Society of Chemistry. It was split into ''Perkin Transactions I'' and ''Perkin Transactions II''. The predecessor journals published by the Chemical Society before the merger of that Society with other Societies to form the Royal Society of Chemistry were the '' Journal of the Chemical Society, Perkin Transactions 1'' and ''Journal of the Chemical Society, Perkin Transactions 2'' (1972-1996). They were replaced by '' Organic and Biomolecular Chemistry''. The name honours the chemist Arthur George Perkin. See also * List of scientific journals in chemistry * List of scientific journals External links * Royal Socie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans. Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature. It is soluble in common organic solvents, including alcohol, ether, and acetone, and is slightly soluble in water. Its odor is "strong, ethereal; chloroform-like". It is toxic and may be carcinogenic in humans. Furan is used as a starting point for other speciality chemicals. History The name "furan" comes from the Latin ''furfur'', which means bran. (Furfural is produced from bran.) The first furan derivative to be described was 2-furoic acid, by Carl Wilhelm Scheele in 1780. Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 and characterised nine years later by John Stenhouse. Furan itself was first prepared by Heinrich Limp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization (HDS) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Congener (chemistry)
In chemistry, congeners are chemical substances "related to each other by origin, structure, or function". Common origin and structure Any significant quantity of a polyhalogenated compound is by default a blend of multiple molecule types because each molecule forms independently, and chlorine and bromine do not strongly select which site(s) they bond to. * Polychlorinated biphenyls (PCBs) are a family of 209 congeners. * Polybrominated biphenyls and polychlorinated diphenyl ethers are also families of 209 congeners. Similarly polychlorinated dibenzodioxins, polychlorinated dibenzofurans, polychlorinated terphenyls, polychlorinated naphthalene, polychloro phenoxy phenol, and polybrominated diphenyl ethers (PBDEs) (pentabromodiphenyl ether, octabromodiphenyl ether, decabromodiphenyl ether), etc. are also groups of congeners. Common origin * Congener (alcohol), substances other than alcohol (desirable or undesirable) also produced during fermentation. *Congeners of oleic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromaticity
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry Of Heterocyclic Compounds
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties of the soil on the moon (cosmochemistry), how medications work (pharmacology), and how to collect DNA evidence at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Diffraction
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among vari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microwave Spectroscopy
Microwave spectroscopy is the spectroscopy method that employs microwaves, i.e. electromagnetic radiation at GHz frequencies, for the study of matter. History The ammonia molecule NH3 is shaped like a pyramid 0.38 Å in height, with an equilateral triangle of hydrogens forming the base.The nitrogen situated on the axis has two equivalent equilibrium positions above and below the triangle of hydrogens, and this raises the possibility of the nitrogen tunneling up and down, through the plane of the H-atoms. In 1932 Dennison et al. ... analyzed the vibrational energy of this molecule and concluded that the vibrational energy would be split into pairs by the presence of these two equilibrium positions. The next year Wright and Randall observed ... a splitting of 0.67 cm–1 in far infrared lines, corresponding to ν = 20 GHz, the value predicted by theory.In 1934 Cleeton and Williams ... constructed a grating echelette spectrometer in order to measure this splitting directly, thereby ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


2.png)
.png)

