|
Sulfur Metabolism
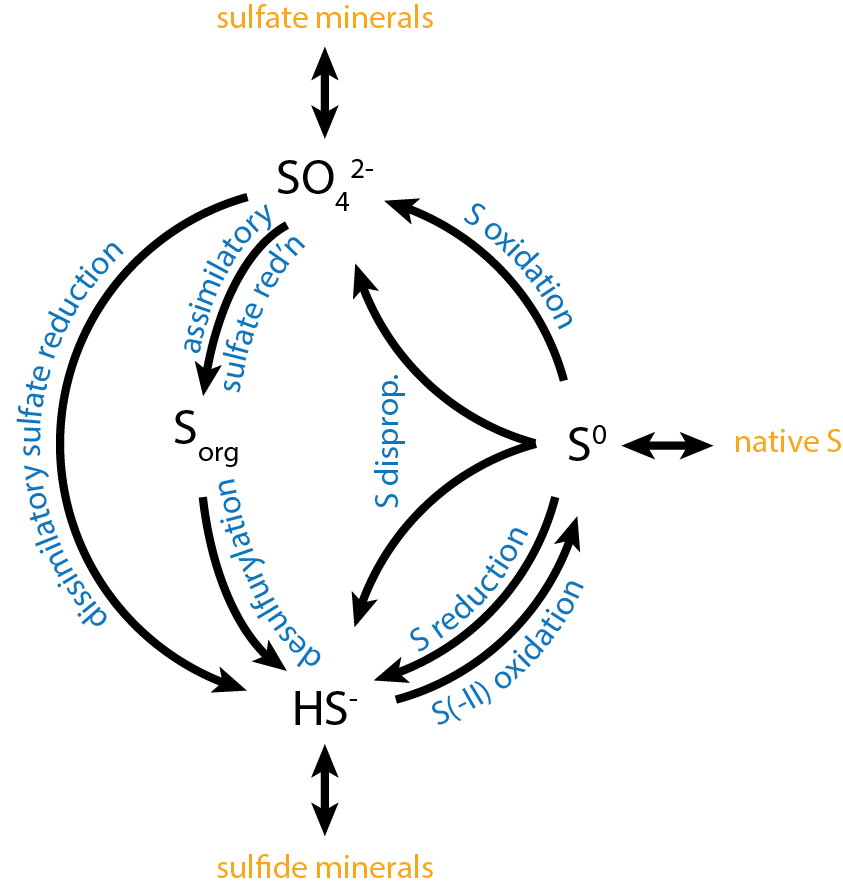
Sulfur is metabolized by all organisms, from bacteria and archaea to plants and animals. Sulfur can have an oxidation state from −2 to +6 and is reduced or oxidized by a diverse range of organisms. The element is present in proteins, sulfate esters of polysaccharides, steroids, phenols, and sulfur-containing coenzymes. Oxidation Reduced sulfur compounds are oxidized by most organisms, including higher animals and higher plants. Some organisms can conserve energy (i.e., produce ATP) from the oxidation of sulfur and it can serve as the sole energy source for some lithotrophic bacteria and archaea. Sulfur oxidizers use enzymes such as Sulfide:quinone reductase, sulfur dioxygenase and sulfite oxidase to oxidize sulfur compounds to sulfate. Sulfur-oxidizing microorganisms Reduced sulfur compounds, such as hydrogen sulfide, elemental sulfur, sulfite, thiosulfate, and various polythionates (e.g., tetrathionate), are oxidized by chemotrophic, phototrophic, and mixotrophic bacteria for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Dioxygenase
Sulfur dioxygenase (, ''sulfur oxygenase'', ''sulfur:oxygen oxidoreductase'') is an enzyme with systematic name ''S-sulfanylglutathione:oxygen oxidoreductase''. This enzyme catalyses the following chemical reaction : sulfur Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ... + O2 + H2O \rightleftharpoons sulfite + 2 H+ (overall reaction) : (1a) glutathione + sulfur \rightleftharpoons S-sulfanylglutathione (spontaneous reaction) : (1b) S-sulfanylglutathione + O2 + H2O \rightleftharpoons glutathione + sulfite + 2 H+ This enzyme contains iron. In humans, sulfur dioxygenase is needed to detoxify sulfide. References External links * {{Portal bar, Biology, border=no EC 1.13.11 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrothermal Vent
Hydrothermal vents are fissures on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hotspots. The dispersal of hydrothermal fluids throughout the global ocean at active vent sites creates hydrothermal plumes. Hydrothermal deposits are rocks and mineral ore deposits formed by the action of hydrothermal vents. Hydrothermal vents exist because the Earth is both geologically active and has large amounts of water on its surface and within its crust. Under the sea, they may form features called black smokers or white smokers, which deliver a wide range of elements to the world's oceans, thus contributing to global marine biogeochemistry. Relative to the majority of the deep sea, the areas around hydrothermal vents are biologically more productive, often hosting complex communities fueled by the chemicals dissolved in the vent fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food Chain
A food chain is a linear network of links in a food web, often starting with an autotroph (such as grass or algae), also called a producer, and typically ending at an apex predator (such as grizzly bears or killer whales), detritivore (such as earthworms and woodlice), or decomposer (such as fungi or bacteria). It is not the same as a food web. A food chain depicts relations between species based on what they consume for energy in trophic levels, and they are most commonly quantified in length: the number of links between a trophic consumer and the base of the chain. Food chain studies play an important role in many biological studies. Food chain stability is very important for the survival of most species. When only one element is removed from the food chain it can result in extinction or immense decreases of survival of a species. Many food chains and food webs contain a keystone species, a species that has a large impact on the surrounding environment and that can directly a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemosynthesis
In biochemistry, chemosynthesis is the biological conversion of one or more carbon-containing molecules (usually carbon dioxide or methane) and nutrients into organic matter using the oxidation of inorganic compounds (e.g., hydrogen gas, hydrogen sulfide) or ferrous ions as a source of energy, rather than sunlight, as in photosynthesis. Chemotroph, Chemoautotrophs, organisms that obtain carbon from carbon dioxide through chemosynthesis, are phylogenetically diverse. Groups that include conspicuous or biogeochemically important taxa include the sulfur-oxidizing Gammaproteobacteria, the Campylobacterota, the Aquificota, the methanogenic archaea, and the neutrophilic iron-oxidizing bacteria. Many microorganisms in dark regions of the oceans use chemosynthesis to produce biomass from single-carbon molecules. Two categories can be distinguished. In the rare sites where hydrogen molecules (H2) are available, the energy available from the reaction between CO2 and H2 (leading to product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acidithiobacillus
''Acidithiobacillus'' is a genus of the '' Acidithiobacillia'' in the phylum "'' Pseudomonadota''". This genus includes ten species of acidophilic microorganisms capable of sulfur and/or iron oxidation: ''Acidithiobacillus albertensis, Acidithiobacillus caldus, Acidithiobacillus cuprithermicus, Acidithiobacillus ferrianus, Acidithiobacillus ferridurans, Acidithiobacillus ferriphilus, Acidithiobacillus ferrivorans, Acidithiobacillus ferrooxidans, Acidithiobacillus sulfuriphilus,'' and ''Acidithiobacillus thiooxidans.'' ''A. ferooxidans'' is the most widely studied of the genus, but ''A. caldus'' and ''A. thiooxidans'' are also significant in research. Like all ''"Pseudomonadota"'', ''Acidithiobacillus'' spp. are Gram-negative and non-spore forming. They also play a significant role in the generation of acid mine drainage; a major global environmental challenge within the mining industry. Some species of ''Acidithiobacillus'' are utilized in bioleaching and biomining. A portion of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemotroph
A chemotroph is an organism that obtains energy by the oxidation of electron donors in their environments. These molecules can be organic ( chemoorganotrophs) or inorganic ( chemolithotrophs). The chemotroph designation is in contrast to phototrophs, which use photons. Chemotrophs can be either autotrophic or heterotrophic. Chemotrophs can be found in areas where electron donors are present in high concentration, for instance around hydrothermal vents. Chemoautotroph Chemoautotrophs are autotrophic organisms that can rely on chemosynthesis, i.e. deriving biological energy from chemical reactions of environmental inorganic substrates and synthesizing all necessary organic compounds from carbon dioxide. Chemoautotrophs can use inorganic energy sources such as hydrogen sulfide, elemental sulfur, ferrous iron, molecular hydrogen, and ammonia or organic sources to produce energy. Most chemoautotrophs are prokaryotic extremophiles, bacteria, or archaea that live in o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Fixation
Biological carbon fixation, or сarbon assimilation, is the Biological process, process by which living organisms convert Total inorganic carbon, inorganic carbon (particularly carbon dioxide, ) to Organic compound, organic compounds. These organic compounds are then used to store energy and as structures for other Biomolecule, biomolecules. Carbon is primarily fixed through photosynthesis, but some organisms use chemosynthesis in the absence of sunlight. Chemosynthesis is carbon fixation driven by chemical energy rather than from sunlight. The process of biological carbon fixation plays a crucial role in the global carbon cycle, as it serves as the primary mechanism for removing from the atmosphere and incorporating it into Biomass (ecology), living biomass. The primary production of organic compounds allows carbon to enter the biosphere. Carbon is considered essential for life as a base element for building organic compounds. The flow of carbon from the Earth's atmosphere, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrathionate
The tetrathionate anion, , is a sulfur oxyanion derived from the compound tetrathionic acid, H2S4O6. Two of the sulfur atoms present in the ion are in oxidation state 0 and two are in oxidation state +5. Alternatively, the compound can be viewed as the adduct resulting from the binding of to SO3. Tetrathionate is one of the polythionates, a family of anions with the formula ''n''(SO3)2sup>2−. Its IUPAC name is ''2-(dithioperoxy)disulfate'', and the name of its corresponding acid is ''2-(dithioperoxy)disulfuric acid''. The Chemical Abstracts Service identifies tetrathionate by the CAS Number 15536-54-6. Formation Tetrathionate is a product of the oxidation of thiosulfate, , by iodine, I2: :2 + I2 → + 2 I− The use of bromine instead of iodine is dubious as excess bromine will oxidize the thiosulfate to sulfate. Structure Tetrathionate's structure can be visualized by following three edges of a rectangular cuboid, as in the diagram below. The structure shown i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, such as sodium thiosulfate and ammonium thiosulfate . Thiosulfate salts occur naturally. Thiosulfate rapidly dechlorinates water, and is used to halt bleaching in the paper-making industry. Thiosulfate salts are mainly used for dyeing in textiles, and bleaching of natural substances. Structure and bonding The thiosulfate ion is tetrahedral at the central S atom. The thiosulfate ion has C3v symmetry. The external sulfur atom has a valence of 2 while the central sulfur atom has a valence of 6. The oxygen atoms have a valence of 2. The S-S distance of about 201 pm in sodium thiosulphate is appropriate for a single bond. The S-O distances are slightly shorter than the S-O distances in sulfate. For many years, the oxidation states of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (systematic name: sulfate(IV) ion), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structure predicted by VSEPR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |