|
Structure Of The Earth
The internal structure of Earth are the layers of the Earth, excluding its atmosphere and hydrosphere. The structure consists of an outer silicate solid crust, a highly viscous asthenosphere, and solid mantle, a liquid outer core whose flow generates the Earth's magnetic field, and a solid inner core. Scientific understanding of the internal structure of Earth is based on observations of topography and bathymetry, observations of rock in outcrop, samples brought to the surface from greater depths by volcanoes or volcanic activity, analysis of the seismic waves that pass through Earth, measurements of the gravitational and magnetic fields of Earth, and experiments with crystalline solids at pressures and temperatures characteristic of Earth's deep interior. Global properties Note: In chondrite model (1), the light element in the core is assumed to be Si. Chondrite model (2) is a model of chemical composition of the mantle corresponding to the model of core shown in chon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Earth Internal Structure
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all of Earth's water is contained in its global ocean, covering Water distribution on Earth, 70.8% of Earth's crust. The remaining 29.2% of Earth's crust is land, most of which is located in the form of continental landmasses within Earth's land hemisphere. Most of Earth's land is at least somewhat humid and covered by vegetation, while large Ice sheet, sheets of ice at Polar regions of Earth, Earth's polar polar desert, deserts retain more water than Earth's groundwater, lakes, rivers, and Water vapor#In Earth's atmosphere, atmospheric water combined. Earth's crust consists of slowly moving tectonic plates, which interact to produce mountain ranges, volcanoes, and earthquakes. Earth's outer core, Earth has a liquid outer core that generates a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Outcrop
An outcrop or rocky outcrop is a visible exposure of bedrock or ancient superficial deposits on the surface of the Earth and other terrestrial planets. Features Outcrops do not cover the majority of the Earth's land surface because in most places the bedrock or superficial deposits are covered by soil and vegetation and cannot be seen or examined closely. However, in places where the overlying cover is removed through erosion or tectonic uplift, the rock may be exposed, or ''crop out''. Such exposure will happen most frequently in areas where erosion is rapid and exceeds the weathering rate such as on steep hillsides, mountain ridges and tops, river banks, and tectonically active areas. In Finland, glacial erosion during the last glacial maximum (ca. 11000 BC), followed by scouring by sea waves, followed by isostatic uplift has produced many smooth coastal and littoral outcrops. Bedrock and superficial deposits may also be exposed at the Earth's surface due to human exca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores. Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classifie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Oxide
Iron(II) oxide or ferrous oxide is the inorganic compound with the formula FeO. Its mineral form is known as wüstite. One of several iron oxides, it is a black-colored powder that is sometimes confused with rust, the latter of which consists of hydrated iron(III) oxide (ferric oxide). Iron(II) oxide also refers to a family of related non-stoichiometric compounds, which are typically iron deficient with compositions ranging from Fe0.84O to Fe0.95O. Preparation FeO can be prepared by the thermal decomposition of iron(II) oxalate. : The procedure is conducted under an inert atmosphere to avoid the formation of iron(III) oxide (). A similar procedure can also be used for the synthesis of manganous oxide and stannous oxide. Stoichiometric FeO can be prepared by heating Fe0.95O with metallic iron at 770 °C and 36 kbar.Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford University Press Reactions FeO is thermodynamically unstable below 575 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single compound calcium oxide. Calcium oxide that survives processing without reacting in building products, such as cement, is called free lime. Quicklime is relatively inexpensive. Both it and the chemical derivative calcium hydroxide (of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Oxide
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several Aluminium oxide (compounds), aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, ALOX or alundum in various forms and applications and alumina is refined from bauxite. It occurs naturally in its crystalline Polymorphism (materials science), polymorphic phase (matter), phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire,which have an alumina content approaching 100%. Al2O3 is used as feedstock to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point. Natural occurrence Corundum is the most common naturally occurring crystallinity, crystalline form of aluminium oxide. ruby, Rubies and sapphires are gem-quality forms o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide (MgO), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from '' magnesia nigra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a mod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolite
Pyrolite is a term used to characterize a model composition of the Earth's Upper mantle (Earth), mantle. This model is based on that a pyrolite source can produce mid-ocean ridge basalts (MORB) by partial melting. It was first proposed by Ted Ringwood (1962) as being 1 part basalt and 4 parts harzburgite, but later was revised to being 1 part tholeiitic basalt and 3 parts dunite. The term is derived from the mineral names Pyroxene, PYR-oxene and Olivine, OL-ivine. However, whether pyrolite is entirely representative of the Earth's mantle remains debated. Chemical composition and phase transition The major elements composition of pyrolite is about 44.71 molar percent (mol%) SiO2, 3.98 % Al2O3, 8.18 % FeO, 3.17 % CaO, 38.73 % MgO, 0.13 % Na2O. 1) A pyrolitic Upper mantle (Earth), Upper Mantle is mainly composed of olivine (~60 volume percent (vol%)), clinopyroxene, orthopyroxene, and garnet. Pyroxene would gradually dissolved into garnet and form Majorite, majoritic garnet. 2) A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Upper Mantle
The upper mantle of Earth is a very thick layer of rock inside the planet, which begins just beneath the crust (geology), crust (at about under the oceans and about under the continents) and ends at the top of the lower mantle (Earth), lower mantle the . Temperatures range from approximately at the upper boundary with the crust to approximately at the boundary with the lower mantle. Upper mantle material that has come up onto the surface comprises about 55% olivine, 35% pyroxene, and 5 to 10% of calcium oxide and aluminum oxide minerals such as plagioclase, spinel, or garnet, depending upon depth. Seismic structure The density profile through Earth is determined by the velocity of seismic waves. Density increases progressively in each layer, largely due to compression of the rock at increased depths. Abrupt changes in density occur where the material composition changes. The upper mantle begins just beneath the crust and ends at the top of the lower mantle. The upper mantl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
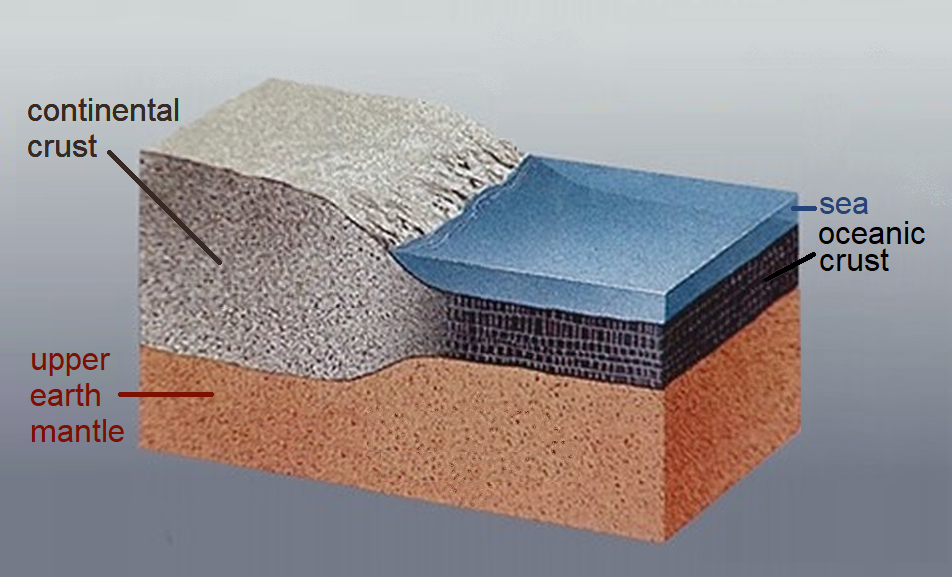
Continental Crust
Continental crust is the layer of igneous, metamorphic, and sedimentary rocks that forms the geological continents and the areas of shallow seabed close to their shores, known as '' continental shelves''. This layer is sometimes called '' sial'' because its bulk composition is richer in aluminium silicates (Al-Si) and has a lower density compared to the oceanic crust, called '' sima'' which is richer in magnesium silicate (Mg-Si) minerals. Changes in seismic wave velocities have shown that at a certain depth (the Conrad discontinuity), there is a reasonably sharp contrast between the more felsic upper continental crust and the lower continental crust, which is more mafic in character. Most continental crust is dry land above sea level. However, 94% of the Zealandia continental crust region is submerged beneath the Pacific Ocean, with New Zealand constituting 93% of the above-water portion. Thickness and density The continental crust consists of various layers, with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |