|
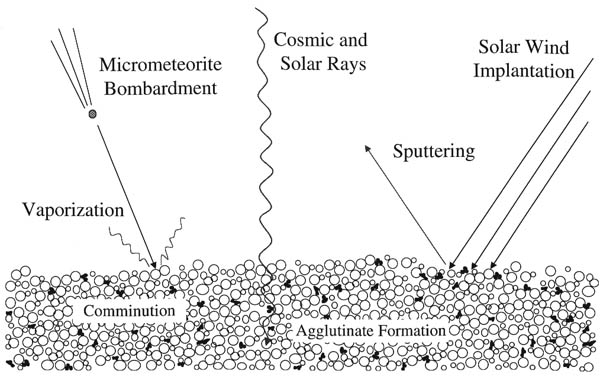
Space Weathering
Space weathering is the type of weathering that occurs to any object exposed to the harsh environment of outer space. Bodies without atmospheres (including the Moon, Mercury, the asteroids, comets, and most of the moons of other planets) take on many weathering processes: * collisions of galactic cosmic rays and solar cosmic rays, * irradiation, implantation, and sputtering from solar wind particles, and * bombardment by different sizes of meteorites and micrometeorites. Space weathering is important because these processes affect the physical and optical properties of the surface of many planetary bodies. Therefore, it is critical to understand the effects of space weathering in order to properly interpret remotely sensed data. History Much of our knowledge of the space weathering process comes from studies of the lunar samples returned by the Apollo program, particularly the lunar soils (or regolith). The constant flux of high energy particles and micrometeorites ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weathering
Weathering is the deterioration of rocks, soils and minerals (as well as wood and artificial materials) through contact with water, atmospheric gases, sunlight, and biological organisms. It occurs '' in situ'' (on-site, with little or no movement), and so is distinct from erosion, which involves the transport of rocks and minerals by agents such as water, ice, snow, wind, waves and gravity. Weathering processes are either physical or chemical. The former involves the breakdown of rocks and soils through such mechanical effects as heat, water, ice and wind. The latter covers reactions to water, atmospheric gases and biologically produced chemicals with rocks and soils. Water is the principal agent behind both kinds, though atmospheric oxygen and carbon dioxide and the activities of biological organisms are also important. Biological chemical weathering is also called biological weathering. The materials left after the rock breaks down combine with organic material to create so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lunar Soil
Lunar regolith is the unconsolidated material found on the selenography, surface of the Moon and in the Lunar atmosphere, Moon's tenuous atmosphere. Sometimes referred to as Lunar soil, Lunar soil specifically refers to the component of regolith smaller than 1 cm. It differs substantially in properties from Soil, terrestrial soil. As the Moon's fine surface layer, lunar regolith is picked up by even weak natural phenomena active at the Moon's surface, allowing it to be part of the Moon's scant atmosphere. It is easily disturbed and poses a significant hazard to exposed equipment and human health. The fine lunar regolith is made of sharp and very adhesive particles, with a distinct gunpowder taste and smell. Lunar regolith is prospected as a Lunar resources, lunar resource, particularly for lunar In situ resource utilization, in situ utilization, such as Lunarcrete, a lunar building material and regolith for Plants in space#Lunar surface, growing plants on the Moon. Lunar rego ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geochimica Et Cosmochimica Acta
for, la, Geochimica et Cosmochimica Acta, Geochemical and Cosmochemical Journal is a biweekly peer-reviewed scientific journal published by Elsevier. It was established in 1950 and is sponsored by the Geochemical Society and the Meteoritical Society. The editor-in-chief is Jeffrey Catalano ( Washington University in St. Louis). The journal covers topics in Earth geochemistry, planetary geochemistry, cosmochemistry and meteoritics. Publishing formats include original research articles and invited reviews and occasional editorials, book reviews, and announcements. In addition, the journal publishes short comments (4 pages) targeting specific articles and designed to improve understanding of the target article by advocating a different interpretation supported by the literature, followed by a response by the author. Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2021 impact factor The imp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patina
Patina ( or ) is a thin layer that variously forms on the surface of copper, brass, bronze, and similar metals and metal alloys ( tarnish produced by oxidation or other chemical processes), or certain stones and wooden furniture (sheen produced by age, wear, and polishing), or any similar acquired change of a surface through age and exposure. Additionally, the term is used to describe the aging of high-quality leather. The patinas on leather goods are unique to the type of leather, frequency of use, and exposure. Patinas can provide a protective covering to materials that would otherwise be damaged by corrosion or weathering. They may also be aesthetically appealing. Usage On metal, patina is a coating of various chemical compounds such as oxides, carbonates, sulfides, or sulfates formed on the surface during exposure to atmospheric elements (oxygen, rain, acid rain, carbon dioxide, sulfur-bearing compounds). Patina also refers to accumulated changes in surface texture and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmission Electron Microscope
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a grid. An image is formed from the interaction of the electrons with the sample as the beam is transmitted through the specimen. The image is then magnified and focused onto an imaging device, such as a fluorescent screen, a layer of photographic film, or a detector such as a scintillator attached to a charge-coupled device or a direct electron detector. Transmission electron microscopes are capable of imaging at a significantly higher resolution than light microscopes, owing to the smaller de Broglie wavelength of electrons. This enables the instrument to capture fine detail—even as small as a single column of atoms, which is thousands of times smaller than a resolvable object seen in a light microscope. Transmission electron micro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Flare
A solar flare is a relatively intense, localized emission of electromagnetic radiation in the Sun's atmosphere. Flares occur in active regions and are often, but not always, accompanied by coronal mass ejections, solar particle events, and other eruptive solar phenomena. The occurrence of solar flares varies with the 11-year solar cycle. Solar flares are thought to occur when stored magnetic energy in the Sun's atmosphere accelerates charged particles in the surrounding plasma. This results in the emission of electromagnetic radiation across the electromagnetic spectrum. The typical time profile of these emissions features three identifiable phases: a ''precursor phase'', an ''impulsive phase'' when particle acceleration dominates, and a ''gradual phase'' in which hot plasma injected into the corona by the flare cools by a combination of radiation and conduction of energy back down to the lower atmosphere. The extreme ultraviolet and X-ray radiation from solar flares is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the Chemical element, elements, and it does not have a melting point at standard pressures. It is the second-lightest and second-most Abundance of the chemical elements, abundant element in the observable universe, after hydrogen. It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and Jupiter, because of the very high nuclear binding energy (per nucleon) of helium-4 with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanophase Material
Nanophase materials are materials that have grain sizes under 100 nanometres. They have different mechanical and optical properties compared to the large grained materials of the same chemical composition. Transparency and different transparent colours can be achieved with nanophase materials by varying the grain size. Nanophase materials *Nanophase metals usually are many times harder but more brittle than regular metals. ** nanophase copper is a superhard material ** nanophase aluminum ** nanophase iron is iron with a grain size in the nanometer range. Nanocrystalline iron has a tensile strength of around 6 GPA, twice that of the best maraging steels. * Nanophase ceramics usually are more ductile and less brittle than regular ceramics. Footnotes External links Creating Nanophase Materials ''Scientific American'' (subscription required) Nanophase Materials ''Michigan Tech'' ''Louisiana State University Louisiana State University and Agricultural and Mechanical Coll ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vaporize
Vaporization (or vapo(u)risation) of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon, whereas boiling is a bulk phenomenon (a phenomenon in which the whole object or substance is involved in the process). Evaporation Evaporation is a phase transition from the liquid phase to vapor (a state of substance below critical temperature) that occurs at temperatures below the boiling temperature at a given pressure. Evaporation occurs ''on the surface''. Evaporation only occurs when the partial pressure of vapor of a substance is less than the equilibrium vapor pressure. For example, due to constantly decreasing pressures, vapor pumped out of a solution will eventually leave behind a cryogenic liquid. Boiling Boiling is also a phase transition from the liquid phase to gas phase, but boiling is the formation of vapor as bubbles of vapor ''below the surface'' of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sputter
In physics, sputtering is a phenomenon in which microscopic particles of a solid material are ejected from its surface, after the material is itself bombarded by energetic particles of a plasma or gas. It occurs naturally in outer space, and can be an unwelcome source of wear in precision components. However, the fact that it can be made to act on extremely fine layers of material is utilised in science and industry—there, it is used to perform precise etching, carry out analytical techniques, and deposit thin film layers in the manufacture of optical coatings, semiconductor devices and nanotechnology products. It is a physical vapor deposition technique. Physics When energetic ions collide with atoms of a target material, an exchange of momentum takes place between them. These ions, known as "incident ions", set off collision cascades in the target. Such cascades can take many paths; some recoil back toward the surface of the target. If a collision cascade reaches the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |