|
Solvothermal Synthesis
Solvothermal synthesis is a method of producing chemical compounds, in which a solvent containing reagents is put under high pressure and temperature in an autoclave. Many substances dissolve better in the same solvent in such conditions than at standard conditions, enabling reactions that would not otherwise occur and leading to new compounds or polymorphs. Solvothermal synthesis is very similar to the hydrothermal route; both are typically conducted in a stainless steel autoclave. The only difference being that the precursor solution is usually non-aqueous. Solvothermal synthesis has been used prepare MOFs, titanium dioxide, and graphene Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ..., carbon spheres, chalcogenides and other materials. Solvents Besides water (hydrothermal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
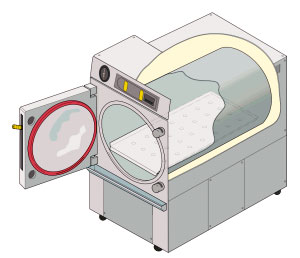
Autoclave 01
An autoclave is a machine used to carry out industrial and scientific processes requiring elevated temperature and pressure in relation to ambient pressure and/or Room temperature, temperature. Autoclaves are used before surgical procedures to perform sterilization (microbiology), sterilization and in the chemical industry to cure coatings and vulcanization, vulcanize rubber and for hydrothermal synthesis. Industrial autoclaves are used in industrial applications, especially in the manufacturing of composites. Many autoclaves are used to sterilize equipment and supplies by subjecting them to pressurized saturated steam at for 30–60 minutes at a gauge pressure of 103 kPa depending on the size of the load and the contents. The autoclave was invented by Charles Chamberland in 1879, although a precursor known as the steam digester was created by Denis Papin in 1679. The name comes from Greek ''auto-'', ultimately meaning self, and Latin ''clavis'' meaning key, thus a self-locking ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, Volatility (chemistry), volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a Precursor (chemistry), precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl ''tert''-butyl ether, methyl benzoate, anisole, peroxyacids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dielectric Constant
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insulator measures the ability of the insulator to store electric energy in an electrical field. Permittivity is a material's property that affects the Coulomb force between two point charges in the material. Relative permittivity is the factor by which the electric field between the charges is decreased relative to vacuum. Likewise, relative permittivity is the ratio of the capacitance of a capacitor using that material as a dielectric, compared with a similar capacitor that has vacuum as its dielectric. Relative permittivity is also commonly known as the dielectric constant, a term still used but deprecated by standards organizations in engineering as well as in chemistry. Definition Relative permittivity is typically denoted as (som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitride
In chemistry, a nitride is a chemical compound of nitrogen. Nitrides can be inorganic or organic, ionic or covalent. The nitride anion, N3−, is very elusive but compounds of nitride are numerous, although rarely naturally occurring. Some nitrides have a found applications, such as wear-resistant coatings (e.g., titanium nitride, TiN), hard ceramic materials (e.g., silicon nitride, Si3N4), and semiconductors (e.g., gallium nitride, GaN). The development of GaN-based light emitting diodes was recognized by the 2014 Nobel Prize in Physics. Metal nitrido complexes are also common. Synthesis of inorganic metal nitrides is challenging because nitrogen gas (N2) is not very reactive at low temperatures, but it becomes more reactive at higher temperatures. Therefore, a balance must be achieved between the low reactivity of nitrogen gas at low temperatures and the entropy driven formation of N2 at high temperatures. However, synthetic methods for nitrides are growing more sophis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imide
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called Polyimide, polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand. Examples Simple example is diacetamide with the formula , formally the diacetylated derivative of ammonia. Commonly encountered imides, however, are cyclic, being derived from dicarboxylic acids. A common example is succinimide derived from succinic acid and ammonia. The names of these cyclic imides reflect the parent acid. Many imides are derived from primary amines as opposed to ammonia. These are indicated by ''N''-substituent in the prefix. For example, N-ethylsuccinimide is derived from succinic acid and ethylamine. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the Polymer backbone, main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a Derivative (chemistry), derivative of a carboxylic acid () with the hydroxyl group () replaced by an amino group (); or, equivalently, an acyl group, acyl (alkanoyl) group () joined to an amino group. Common amides are formamide (), acetamide (), benzamide (), and dimethylformamide (). Some uncommon examples of amides are ''N''-chloroacetamide () and chloroformamide (). Amides are qualified as primary (chemistry), primary, secondary (chemistry), secondary, and tertiary (chemistry), tertiary according to the number of acyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group . The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, , the chief constituent of limestone (as well as the main component of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of (called a passivation layer) that protects the foil from further oxidation.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry Oxides are extraordinarily diverse in terms of stoichiometries (the measurable relationship between reactants and chemical equations of an equation or reaction) and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnsh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest oxocarbon, carbon oxide. In coordination complexes, the carbon monoxide ligand is called ''metal carbonyl, carbonyl''. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds. Numerous environmental and biological sources generate carbon monoxide. In industry, carbon monoxide is important in the production of many compounds, including drugs, fragrances, and fuels. Indoors CO is one of the most acutely toxic contaminants affecting indoor air quality. CO may be emitted from tobacco smoke and generated from malfunctioning fuel-burning stoves (wood, kerosene, natural gas, propane) and fuel-burning heating systems (wood, oil, n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formic Acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Esters, salts, and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol. Natural occurrence Formic acid, which has a pungent, penetrating odor, is found naturally in insects, weeds, fruits and vegetables, and forest emissions. It appears in most ants and in stingless bees of the genus '' Oxytrigona''. Wood ants from the genus ''Formica'' can spray formic acid on their prey or to defend the nest. The puss moth caterpillar (''Cerura vinula'') will spray it as well when threatened by predators. It is also found in the trichomes of stinging nettle (''Urtica dioica''). Apart from that, this acid is incorporated in many fruits such as pineapple (0.21 mg per 100 g), apple (2 mg per ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexane-1,6-diol
1,6-Hexanediol is an organic compound with the formula (CH2CH2CH2OH)2. It is a colorless water-soluble solid. History and synthesis W. H. Perkin Jr. and his graduate student Edward Haworth first prepared the compound in 1894 during their research on cyclohexane. They boiled 1,6-dibromohexane (which they were also the first to synthesize) with dilute potassium carbonate solution and named the product hexamethylene glycol. 1,6-Hexanediol is industrially made by the hydrogenation of adipic acid or its esters. Laboratory preparation could be achieved by reduction of adipates with lithium aluminium hydride, although this method is impractical on a commercial scale. Properties As 1,6-hexanediol contains hydroxyl groups, it undergoes the typical chemical reactions of alcohols such as dehydration, substitution, and esterification. Oxidation with pyridinium chlorochromate gives adipaldehyde. Dehydration of 1,6-hexanediol gives oxepane, 2-methyltetrahydropyran and 2-ethyltetrahydrof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |