|
Sedaxane
Sedaxane is a broad spectrum fungicide used as a seed treatment in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2011 using their brand name Vibrance. The compound is an amide which combines a pyrazole acid with an aryl amine to give an inhibitor of succinate dehydrogenase. The compound is widely registered for use, including in Australia, the EU, UK and US. History Inhibition of succinate dehydrogenase, the complex II in the mitochondrial respiration chain, has been known as a fungicidal mechanism of action since the first examples were marketed in the 1960s. The first compound in this class was carboxin, which had a narrow spectrum of useful biological activity, mainly on basidiomycetes and was used as a seed treatment. By 2016, at least 17 further examples of this mechanism of action were developed by crop protection companies, with the market leader being boscalid, owing to its broader spectrum of fungal species controlled. However, it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic Acid
3-(Difluoromethyl)-1-methyl-1''H''-pyrazole-4-carboxylic acid is a chemical compound which is used commercially as an intermediate to seven fungicides which act by inhibition of succinate dehydrogenase (SDHI). It consists of a pyrazole ring with difluoromethyl, methyl and carboxylic acid groups attached in specific positions. Background Inhibition of succinate dehydrogenase, the complex II in the mitochondrial respiration chain, has been known as a fungicidal mechanism of action since the first examples were marketed in the 1960s. By 2016, at least 18 examples were developed by crop protection companies, with the market leader being boscalid, owing to its broad spectrum of fungal species controlled. However, it lacked full control of important cereal diseases, especially septoria leaf blotch ''Zymoseptoria tritici''. A group of compounds which did control septoria were 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic amides, as shown below, ordered by year of their first reg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seed Treatment
In agriculture and horticulture, a seed treatment is any additional material added to the seed. By the amount of material added, it can be divided into: * A seed dressing, typically containing a "protectant" ( pesticide) applied to the seed and possibly some color. * A seed coating, a layer of thin film applied to the seed typically less than 10% of the mass of the original seed. * Seed encrusting, where the applied material is typically 100%–500% of the original seed mass, but the shape is still discernible. * Seed pelleting, where the applied material is so thick that the seed's original shape is not discernible. Seed treatment provides the following functions: * For formulations with pesticides, direct application to seeds can be environmentally more friendly, as the amounts used can be very small. * Color makes treated seed less attractive to birds, and easier to see and clean up in the case of an accidental spillage. * A thick coating can improve handling, by hand or by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
British Crop Production Council
The British Crop Production Council (BCPC) is an organisation that promotes the use of good science and technology in the understanding and application of effective and sustainable crop production. BCPC is a Registered Charity and a Company limited by Guarantee. Function The key objectives of BPCP are to: *Identify developing issues in the science and practice of crop protection and production, and provide informed, independent analysis and views on these to opinion formers, government and the public *Publish definitive information for growers, advisors and other stakeholders in the food, fuel and fibre production chain, in the form of reference works, manuals and handbooks *Organise and co-host conferences and symposia to provide platforms for the reporting and debate of scientific relevant results and opinion *Contribute to the future of UK (bio) science by providing publications for schools which stimulate interest and learning. History BCPC was formed in 1967 by the amalga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomers
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation will allow the four unique groups on the chiral carbon (see Chirality (chemistry)) to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are non-superposable onto each other however, they are not mirror images of each other. A molecule with chirality rotates plane-polarized light. A mixture of equals amo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diastereomers
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two. Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image (that is, excluding the opposing enantiomer). Diastereomers h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of a pre-existing one. The selectivity arises from differences in steric and electronic effects in the mechanistic pathways leading to the different products. Stereoselectivity can vary in degree but it can never be total since the activation energy difference between the two pathways is finite. Both products are at least possible and merely differ in amount. However, in favorable cases, the minor stereoisomer may not be detectable by the analytic methods used. An enantioselective reaction is one in which one enantiomer is formed in preference to the other, in a reaction that creates an optically active product from an achiral starting material, using either a chiral catalyst, an enzyme or a chiral reagent. The degree of selectivity is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzophenone Imine
Benzophenone imine is an organic compound with the formula of (C6H5)2C=NH. A pale yellow liquid, benzophenone imine is used as a reagent in organic synthesis. Synthesis Benzophenone imine can be prepared by the thermal decomposition of benzophenone oxime: :2(C6H5)2C=NOH → (C6H5)2C=NH + (C6H5)2C=O Benzophenone imine can also be synthesized by addition of phenylmagnesium bromide to benzonitrile followed by careful hydrolysis (lest the imine be hydrolyzed): :C6H5CN + C6H5MgBr → (C6H5)2C=NMgBr :(C6H5)2C=NMgBr + H2O → (C6H5)2C=NH + MgBr(OH) This method is known as Moureu-Mignonac ketimine synthesis. Yet another route to benzophenone imine involves reaction of benzophenone and ammonia. Reactions Benzophenone imine undergoes deprotonation with alkyl lithium reagents. :(C6H5)2C=NH + CH3Li → (C6H5)2C=NLi + CH4 :(C6H5)2C=NLi + CH3I → (C6H5)2C=NCH3 + LiI Primary amines can be protected as benzophenone imines, and the protected amines are stable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
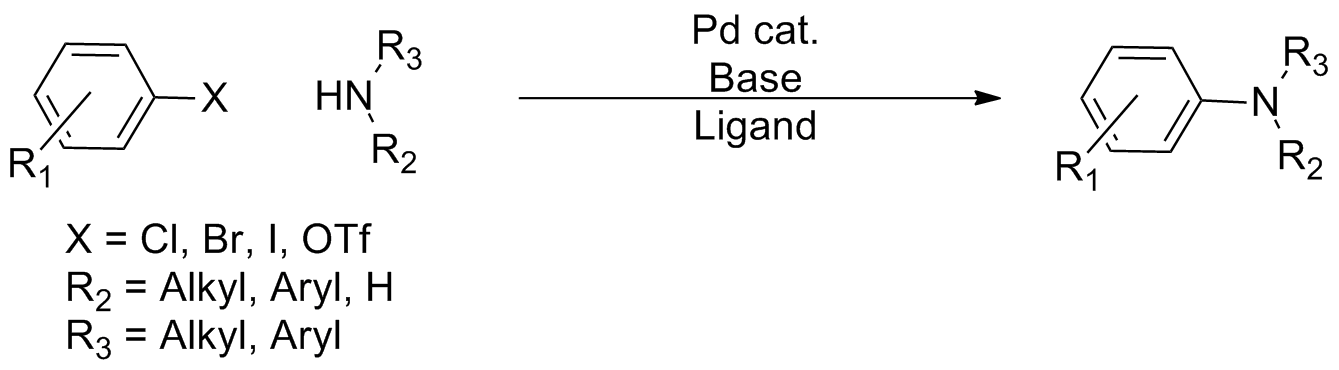
Buchwald–Hartwig Amination
In organic chemistry, the Buchwald–Hartwig amination is a chemical reaction for the synthesis of carbon–nitrogen bonds via the palladium-catalyzed coupling reactions of amines with aryl halides. Although Pd-catalyzed C-N couplings were reported as early as 1983, Stephen L. Buchwald and John F. Hartwig have been credited, whose publications between 1994 and the late 2000s established the scope of the transformation. The reaction's synthetic utility stems primarily from the shortcomings of typical methods ( nucleophilic substitution, reductive amination, etc.) for the synthesis of aromatic bonds, with most methods suffering from limited substrate scope and functional group tolerance. The development of the Buchwald–Hartwig reaction allowed for the facile synthesis of aryl amines, replacing to an extent harsher methods (the Goldberg reaction, nucleophilic aromatic substitution, etc.) while significantly expanding the repertoire of possible bond formation. : Over the co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash. Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exploit its caustic nature and its reactivity toward acids. An estimated 700,000 to 800,000 tonnes were produced in 2005. KOH is noteworthy as the precursor to most soft and liquid soaps, as well as numerous potassium-containing chemicals. It is a white solid that is dangerously corrosive. Properties and structure KOH exhibits high thermal stability. Because of this high stability and relatively low melting point, it is often melt-cast as pellets or rods, forms that have low surface area and convenient handling properties. These pellets become tacky in air because KOH is hygroscopic. Most commercial samples are ca. 90% pure, the remainder being water and carbonates. Its dissolution in water is strongly exothermic. Concentrated aqueous so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in- space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |