|
SeF4
Selenium tetrafluoride ( Se F4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas. Synthesis The first reported synthesis of selenium tetrafluoride was by Paul Lebeau in 1907, who treated selenium with fluorine: :Se + 2 F2 → SeF4 A synthesis involving more easily handled reagents entails the fluorination of selenium dioxide with sulfur tetrafluoride: :SF4 + SeO2 → SeF4 + SO2 An intermediate in this reaction is seleninyl fluoride (SeOF2). Other methods of preparation include fluorinating elemental selenium with chlorine trifluoride: :3 Se + 4 ClF3 → 3 SeF4 + 2 Cl2 Structure and bonding Selenium in SeF4 has an oxidation state of +4. Its shape in the gaseous phase is similar to that of SF4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium Dioxide
Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium. It is used in making specialized glasses as well as a reagent in organic chemistry. Properties Solid SeO2 is a one-dimensional polymer, the chain consisting of alternating selenium and oxygen atoms. Each Se atom is pyramidal and bears a terminal oxide group. The bridging Se-O bond lengths are 179 pm and the terminal Se-O distance is 162 pm.Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium, Franceso A. Devillanova, Royal Society of Chemistry, 2007, The relative stereochemistry at Se alternates along the polymer chain ( syndiotactic). In the gas phase selenium dioxide is present as dimers and other oligomeric species, at higher temperatures it is monomeric. The monomeric form adopts a bent structure very similar to that of sulfur dioxide with a bond length of 161 pm. The dimeric form has been iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
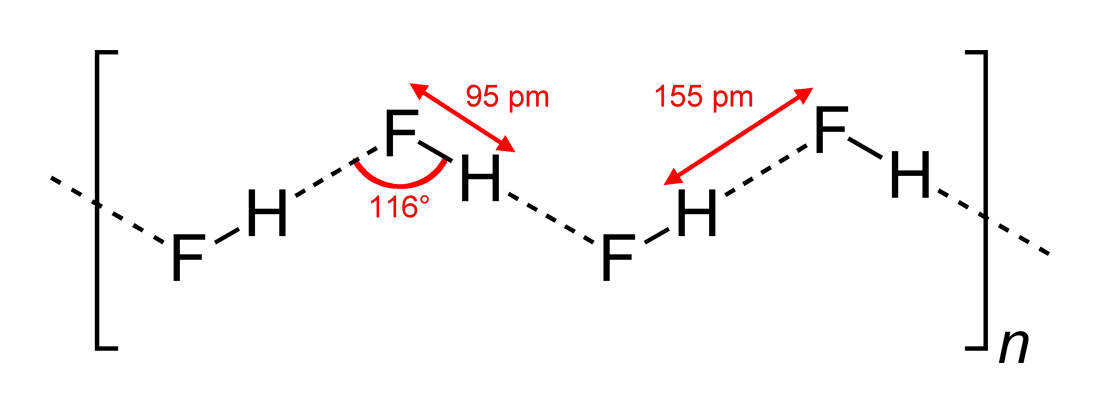
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium(IV) Compounds
Selenium is a chemical element; it has symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elemental state or as pure ore compounds in Earth's crust. Selenium ( ) was discovered in 1817 by , who noted the similarity of the new element to the previously discovered tellurium (named for the Earth). Selenium is found in metal sulfide ores, where it substitutes for sulfur. Commercially, selenium is produced as a byproduct in the refining of these ores. Minerals that are pure selenide or selenate compounds are rare. The chief commercial uses for selenium today are glassmaking and pigments. Selenium is a semiconductor and is used in photocells. Applications in electronics, once important, have been mostly replaced with silicon semiconductor devices. Selenium is still used in a few types of DC power surge protectors and one type of fluorescent quantum dot. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorides
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic fluoride and those in which fluoride does not dissociate. The nome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tellurium Tetrafluoride
Tellurium tetrafluoride, TeF4, is a stable, white, hygroscopic crystalline solid and is one of two fluorides of tellurium. The other binary fluoride is tellurium hexafluoride.''Inorganic Chemistry'',Egon Wiberg, Arnold Frederick Holleman Elsevier 2001 The widely reported Te2F10 has been shown to be F5TeOTeF5 There are other tellurium compounds that contain fluorine, but only the two mentioned contain solely tellurium and fluorine. Tellurium difluoride, TeF2, and ditellurium difluoride, Te2F2 are not known. Preparation Tellurium tetrafluoride can be prepared by the following reaction: : TeO2 + 2 SF4 → TeF4 + 2 SOF2 It is also prepared by reacting nitryl fluoride with tellurium or from the elements at 0 °C or by reacting selenium tetrafluoride with tellurium dioxide at 80 °C. Fluorine in nitrogen can react with TeCl2 or TeBr2 to form TeF4. PbF2 will also fluorinate tellurium to TeF4. Reactivity Tellurium tetrafluoride will react with water or silica and for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
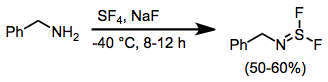
Sulfur Tetrafluoride
Sulfur tetrafluoride is a chemical compound with the formula S F4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries. Structure Sulfur in SF4 is in the +4 oxidation state, with one lone pair of electrons. The atoms in SF4 are arranged in a see-saw shape, with the sulfur atom at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium Hexafluoride
Selenium hexafluoride is the inorganic compound with the formula SeF6. It is a very toxic colourless gas described as having a "repulsive" odor. It is not widely encountered and has no commercial applications. Structure, preparation, and reactions SeF6 has octahedral molecular geometry with an Se−F bond length of 168.8 pm. In terms of bonding, it is hypervalent. SeF6 can be prepared from the elements. It also forms by the reaction of bromine trifluoride (BrF3) with selenium dioxide. The crude product can be purified by sublimation. The relative reactivity of the hexafluorides of S, Se, and Te follows the order TeF6 > SeF6 > SF6, the latter being completely inert toward hydrolysis until high temperatures. SeF6 also resists hydrolysis. The gas can be passed through 10% NaOH or KOH without change, but reacts with gaseous ammonia at 200 °C. Safety Although selenium hexafluoride is quite inert and slow to hydrolyze, it is toxic even at low concentrations, especiall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature (journal)
''Nature'' is a British weekly scientific journal founded and based in London, England. As a multidisciplinary publication, ''Nature'' features Peer review, peer-reviewed research from a variety of academic disciplines, mainly in science and technology. It has core editorial offices across the United States, continental Europe, and Asia under the international scientific publishing company Springer Nature. ''Nature'' was one of the world's most cited scientific journals by the Science Edition of the 2022 ''Journal Citation Reports'' (with an ascribed impact factor of 50.5), making it one of the world's most-read and most prestigious academic journals. , it claimed an online readership of about three million unique readers per month. Founded in the autumn of 1869, ''Nature'' was first circulated by Norman Lockyer and Alexander MacMillan (publisher), Alexander MacMillan as a public forum for scientific innovations. The mid-20th century facilitated an editorial expansion for the j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The Chemical Society
The ''Journal of the Chemical Society'' was a scientific journal established by the Chemical Society in 1849 as the ''Quarterly Journal of the Chemical Society''. The first editor was Edmund Ronalds. The journal underwent several renamings, splits, and mergers throughout its history. In 1980, the Chemical Society merged with several other organizations into the Royal Society of Chemistry. The journal's continuity is found in '' Chemical Communications'', '' Dalton Transactions'', '' Faraday Transactions'', and '' Perkin Transactions'', all of which are published by the Royal Society of Chemistry. History ;'' Proceedings of the Chemical Society'' * ''Memoirs of the Chemical Society of London'' (1841) * ''Proceedings of the Chemical Society of London'' (1842–1843) * ''Memoirs and Proceedings of the Chemical Society'' (1843–1848) * ''Proceedings of the Chemical Society, London'' (1885–1914) * Published as a supplement to ''Journal of the Chemical Society'' from 1914 to 1956 * ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine Pentafluoride
Bromine pentafluoride, Br F5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent. BrF5 finds use in oxygen isotope analysis. Laser ablation of solid silicates in the presence of BrF5 releases O2 for subsequent analysis. It has also been tested as an oxidizer in liquid rocket propellants and is used as a fluorinating agent in the processing of uranium. Preparation BrF5 was first prepared in 1931 by the direct reaction of bromine and fluorine. This reaction is suitable for the preparation of large quantities, and is carried out at temperatures over with an excess of fluorine: :Br2 + 5 F2 → 2 BrF5 For the preparation of smaller amounts, potassium bromide is used: :KBr + 3 F2 → KF + BrF5 This route yields BrF5 almost completely free of trifluorides and other impurities. Reactions BrF5 reacts with water to form bromic acid and hydrofluoric acid: :BrF5 + 3 H2O → HBrO3 + 5 HF It is an extremely effective fluorinating agent, bei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |