Sulfur tetrafluoride on:
[Wikipedia]
[Google]
[Amazon]
Sulfur tetrafluoride is a
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the formula S F4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.
Structure
Sulfur in SF4 is in the +4 oxidation state, with one lone pair of electrons. The atoms in SF4 are arranged in a see-saw shape, with the sulfur atom at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation.Synthesis and manufacture
At the laboratory scale, sulfur tetrafluoride is prepared from elemental sulfur and cobaltic fluoride :S + 4CoF3 → SF4 + 4CoF2 SF4 is industrially produced by the reaction of SCl2 and NaF with acetonitrile as a catalyst :3 SCl2 + 4 NaF → SF4 + S2Cl2 + 4 NaCl At higher temperatures (e.g. 225–450 °C), the solvent is superfluous. Moreover, sulfur dichloride may be replaced by elemental sulfur (S) and chlorine (Cl2). A low-temperature (e.g. 20–86 °C) alternative to the chlorinative process above uses liquid bromine (Br2) as oxidant and solvent: :S(s) + 2 Br2(l; excess) + 4KF(s) → SF4↑ + 4 KBr(brom)Use in synthesis of organofluorine compounds
In organic synthesis, SF4 is used to convert COH and C=O groups into CF and CF2 groups, respectively. The efficiency of these conversions are highly variable. In the laboratory, the use of SF4 has been superseded by the safer and more easily handled diethylaminosulfur trifluoride, (C2H5)2NSF3, "DAST": This reagent is prepared from SF4: :Other reactions
Sulfur chloride pentafluoride (), a useful source of the SF5 group, is prepared from SF4. : Hydrolysis of SF4 givessulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
:
:SF4 + 2 H2O → SO2 + 4 HF
This reaction proceeds via the intermediacy of thionyl fluoride, which usually does not interfere with the use of SF4 as a reagent.
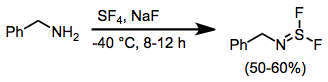
When amines are treated with SF4 and a base, aminosulfur difluorides result.

Toxicity
reacts inside the lungs with moisture, formingsulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
and hydrogen fluoride which forms highly toxic and corrosive hydrofluoric acid
References
{{Sulfur compounds Sulfur fluorides Fluorinating agents Hypervalent molecules ja:フッ化硫黄#四フッ化硫黄