|
SbF3
Antimony trifluoride is the inorganic compound with the formula SbF3. Sometimes called Swarts' reagent, it is one of two principal fluorides of antimony, the other being SbF5. It appears as a white solid. As well as some industrial applications, it is used as a reagent in inorganic and organofluorine chemistry. Preparation and structure In solid SbF3, the Sb centres have octahedral molecular geometry and are linked by bridging fluoride ligands. Three Sb–F bonds are short (192 pm) and three are long (261 pm). Because it is a polymer, SbF3 is far less volatile than related compounds AsF3 and SbCl3. SbF3 is prepared by treating antimony trioxide with hydrogen fluoride: :Sb2O3 + 6 HF → 2 SbF3 + 3 H2O The compound is a mild Lewis acid, hydrolyzing slowly in water. With fluorine, it is oxidized to give antimony pentafluoride. :SbF3 + F2 → SbF5 Applications It is used as a fluorination reagent in organic chemistry. This application was reported by the Belgian c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony(III) Compounds
Antimony is a chemical element; it has symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name kohl. The earliest known description of this metalloid in the West was written in 1540 by Vannoccio Biringuccio. China is the largest producer of antimony and its compounds, with most production coming from the Xikuangshan Mine in Hunan. The industrial methods for refining antimony from stibnite are roasting followed by reduction with carbon, or direct reduction of stibnite with iron. The most common applications for metallic antimony are in alloys with lead and tin, which have improved properties for solders, bullets, and plain bearings. It improves the rigidity of lead-alloy plates in lead–acid batteries. Antimony trioxide is a prominent additive for halogen-con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name Kohl (cosmetics), kohl. The earliest known description of this metalloid in the West was written in 1540 by Vannoccio Biringuccio. China is the largest producer of antimony and its compounds, with most production coming from the Xikuangshan Mine in Hunan. The industrial methods for refining antimony from stibnite are Roasting (metallurgy), roasting followed by carbothermic reaction, reduction with carbon, or direct reduction of stibnite with iron. The most common applications for metallic antimony are in alloys with lead and tin, which have improved properties for solders, Bullet, bullets, and plain bearings. It improves the rigidity of lead-alloy pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, Volatility (chemistry), volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a Precursor (chemistry), precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl ''tert''-butyl ether, methyl benzoate, anisole, peroxyacids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
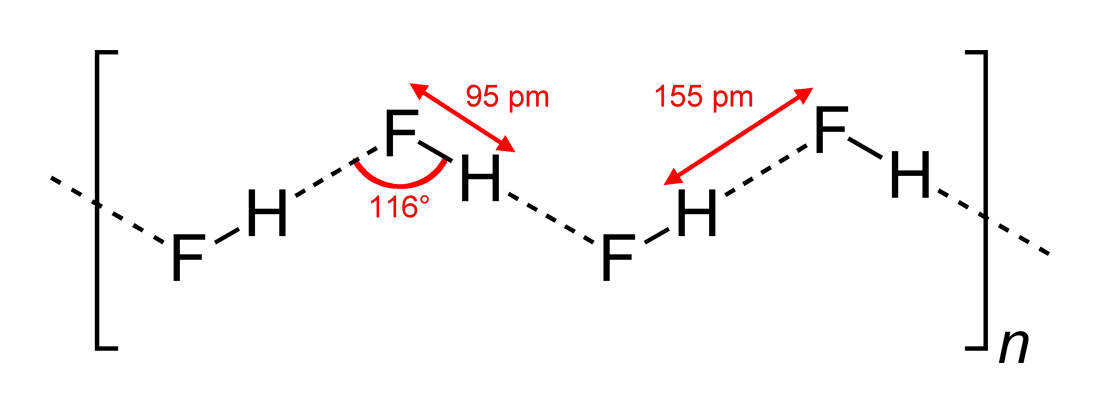
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pottery
Pottery is the process and the products of forming vessels and other objects with clay and other raw materials, which are fired at high temperatures to give them a hard and durable form. The place where such wares are made by a ''potter'' is also called a ''pottery'' (plural ''potteries''). The definition of ''pottery'', used by the ASTM International, is "all fired ceramic wares that contain clay when formed, except technical, structural, and refractory products". End applications include tableware, ceramic art, decorative ware, toilet, sanitary ware, and in technology and industry such as Insulator (electricity), electrical insulators and laboratory ware. In art history and archaeology, especially of ancient and prehistoric periods, pottery often means only vessels, and sculpture, sculpted figurines of the same material are called terracottas. Pottery is one of the Timeline of historic inventions, oldest human inventions, originating before the Neolithic, Neolithic period, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acids
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane CH3)3Bis a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as pos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichlorodifluoromethane
Dichlorodifluoromethane (R-12) is a colorless gas popularly known by the genericized brand name Freon (as Freon-12). It is a chlorofluorocarbon halomethane (CFC) used as a refrigerant and aerosol spray propellant. In compliance with the Montreal Protocol, its manufacture was banned in developed countries (non-article 5 countries) in 1996, and in developing countries (Article 5 countries) in 2010 out of concerns about its damaging effect on the ozone layer. Its only allowed usage is as a fire retardant in submarines and aircraft. It is soluble in many organic solvents. R-12 cylinders are colored white. Preparation It can be prepared by reacting carbon tetrachloride with hydrogen fluoride in the presence of a catalytic amount of antimony pentachloride: :CCl4 + 2HF → CCl2F2 + 2HCl This reaction can also produce trichlorofluoromethane (CCl3F), chlorotrifluoromethane (CClF3) and tetrafluoromethane (CF4). History Charles F. Kettering, vice president of General Motors Researc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silane
Silane (Silicane) is an inorganic compound with chemical formula . It is a colorless, pyrophoric gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silanes with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. They are commonly used to apply coatings to surfaces or as an adhesion promoter. Production Commercial-scale routes Silane can be produced by several routes. Typically, it arises from the reaction of hydrogen chloride with magnesium silicide: : It is also prepared from metallurgical-grade silicon in a two-step process. First, silicon is treated with hydrogen chloride at about 300 °C to produce trichlorosilane, HSiCl3, along with hydrogen gas, according to the chemical equation : The trichlorosilane is then converted to a mixture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorine Compound
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/mol). This is significantly st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Swarts Reaction
Swarts fluorination is a process whereby the chlorine atoms in a compound – generally an organic compound, but experiments have been performed using silanes – are replaced with fluorine, by treatment with antimony trifluoride in the presence of chlorine or of antimony pentachloride. Some metal fluorides are particularly more useful than others, including silver(I) fluoride, mercurous fluoride, cobalt(II) fluoride and aforementioned antimony. Heating the mixture of the metal fluoride and the haloalkane (chlorine and bromine are replaced readily) yields the desired fluoroalkane. In some particularly reactive cases, heating is unnecessary; shaking or stirring the reaction mixture is sufficient. This reaction has a good yield. The process was initially described by Frédéric Jean Edmond Swarts in 1892. Mechanism The active species is antimony trifluorodichloride 2, which is produced in situ is a Latin phrase meaning 'in place' or 'on site', derived from ' ('in') and ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony Pentachloride
Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers. Preparation and structure Antimony pentachloride is prepared by passing chlorine gas into molten antimony trichloride: :SbCl3 + Cl2 → SbCl5 Gaseous SbCl5 has a trigonal bipyramidal structure. Reactions This compounds reacts with water to form antimony pentoxide and hydrochloric acid: :2 SbCl5 + 5 H2O → Sb2O5 + 10 HCl The mono- and tetrahydrates are known, SbCl5·H2O and SbCl5·4H2O. This compound forms adducts with many Lewis bases. SbCl5 is a soft Lewis acid and its ECW model parameters are EA = 3.64 and CA = 10.42. It is used as the standard Lewis acid in the Gutmann scale of Lewis basicity. It is also a strong oxidizing agent. For example aromatic ethers are oxidize ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidizing agent, oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and . However, the nature of fre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |