|
Sarcotoxin
Sarcotoxins are a group of antibacterial peptides present in the flesh fly belonging to the genus ''Sarcophaga''. The proteins are present in the haemolymph of the flesh fly. The first protein, called sarcotoxin 1A, was discovered in 1983 from '' Sarcophaga peregrina'' by Masayuki Okada and Shunji Natori at the University of Tokyo, Japan. The name Sarcotoxin is derived from the peptide's discovery in ''Sarcophaga'' flies, and so antibacterial compounds were given the name Sarcotoxin and an identifying number or letter. However many Sarcotoxins are homologues of Cecropin Cecropins are antimicrobial peptides. They were first isolated from the hemolymph of '' Hyalophora cecropia'', whence the term cecropin was derived. Cecropins lyse bacterial cell membranes; they also inhibit proline uptake and cause leaky membran ...s or Attacins, and are not unique to ''Sarcophaga'' flies. References {{Reflist External linksDescription at COPE [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarcophaga Peregrina
''Sarcophaga peregrina'' (synonym ''Boettcherisca peregrina'') is a species of flesh fly belonging to the family Sarcophagidae. They easily breed, multiply and spread in human habitation, from garbage, faeces and livestock manures. In many regions, they are health concerns as they are active vectors of infectious diseases such as myiasis in humans. Due to their close contact with human activities, they are considered as forensically important insects. They can be used for molecular analysis of the time of postmortem intervals. They are also occasionally parasitic in other invertebrates. They produce a group of antibacterial peptide called sarcotoxins. The first of such protein, sarcotoxin 1A, was determined in 1983 by Masayuki Okada and Shunji Natori at the University of Tokyo, Japan. Distribution ''Sarcophaga peregrina'' has been reported in Southeast Asian region from Sri Lanka to Indonesia, China, Japan, Australia, Samoa and Cook Islands, Hawaii, and throughout Europe. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cecropin
Cecropins are antimicrobial peptides. They were first isolated from the hemolymph of '' Hyalophora cecropia'', whence the term cecropin was derived. Cecropins lyse bacterial cell membranes; they also inhibit proline uptake and cause leaky membranes. Cecropins constitute a main part of the innate immune system of insects. Cecropins are small proteins anywhere from 31 - 37 amino acids long and are active against both gram-positive and gram-negative bacteria. Cecropins isolated from insects other than '' Hyalophora cecropia'' (Cecropia moth) have been given various names, such as bactericidin, lepidopterin, and sarcotoxin. All of these peptides are structurally related. Members Members include: ;Cecropin A: Peptide Sequence (KWKLFKKIEKVGQNIRDGIIKAGPAVAVVGQATQIAK). Secondary structure includes two α helices. At low peptide to lipid ratios ion channels are formed, at high peptide to lipid ratios pores are formed. ;Cecropin B: Peptide Sequence (KWKVFKKIEKMGRNIRNGIVKAGPAIAVLGEAKAL) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
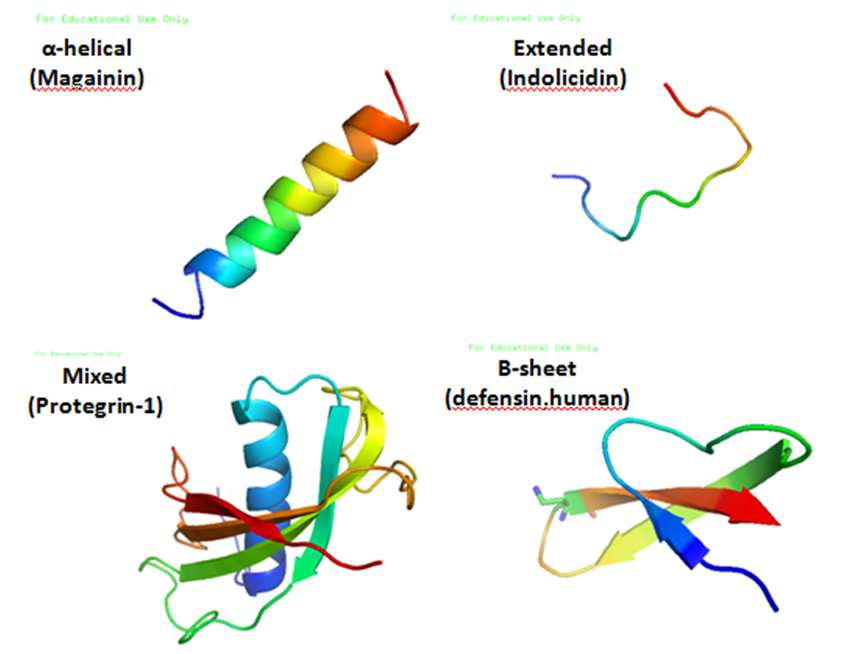
Antibacterial Peptides
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators. Structure Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. Antimicrobial peptides are g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarcophaga
''Sarcophaga'' is a genus of true flies and the type genus of the flesh-fly family (Sarcophagidae). The members of this cosmopolitan genus are frequently known as common flesh flies. This genus occurs essentially worldwide. These flies are generally well-sized and of a greyish color; like many of their relatives, the typical patterns are lengthwise darker stripes on the thorax and dark and light square dots on the abdomen. Many have conspicuous red compound eyes. These are set further apart in females than in males; the females are also larger on average. As typical for this family, it is almost impossible to tell the species apart from their outward appearance, and many can only be reliably identified by microscopic examination of the males' genitalia. As the common name implies, their larvae typically feed on decaying meat. Some, however, instead eat the bacteria and other small organisms living on carrion. Many species have adapted to humans, and while they are usually nuis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Attacin
Attacin is a glycine-rich protein of about 20 kDa belonging to the group of antimicrobial peptides (AMP). It is active against Gram-negative bacteria. Attacin was first discovered in '' Hyalophora cecropia'', but is widely conserved in different insects from butterflies to fruit flies. See also *Diptericin Diptericin is a 9 kDa antimicrobial peptide (AMP) of flies first isolated from the blowfly '' Phormia terranova''. It is primarily active against Gram-negative bacteria, disrupting bacterial membrane integrity. The structure of this protein incl ..., a structurally related antimicrobial peptide References {{Reflist Insect immunity Antimicrobial peptides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimicrobial Peptides
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators. Structure Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. Antimicrobial peptides a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |