|
SNi
In chemistry, Si (substitution nucleophilic internal) refers to a specific, regio-selective but not often encountered reaction mechanism for nucleophilic aliphatic substitution. The name was introduced by Cowdrey et al. in 1937 to label nucleophilic reactions which occur with retention of configuration, but later was employed to describe various reactions that proceed with a similar mechanism. A typical representative organic reaction displaying this mechanism is the chlorination of alcohols with thionyl chloride, or the decomposition of alkyl chloroformates, the main feature is retention of stereochemical configuration. Some examples for this reaction were reported by Edward S. Lewis and Charles E. Boozer in 1952. Mechanistic and kinetic studies were reported few years later by various researchers. Thionyl chloride first reacts with the alcohol to form an alkyl chloro sulfite, actually forming an intimate ion pair. The second step is the loss of a sulfur dioxide molecule ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thionyl Chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately Volatility (chemistry), volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a Halogenation, chlorinating reagent, with approximately per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also List of Schedule 3 substances (CWC), listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons. Thionyl chloride is sometimes confused with sulfuryl chloride, , but the properties of these compounds differ significantly. Sulfuryl chloride is a source of chlorine whereas thionyl chloride is a source of chloride ions. Production The major industrial synthesis involves the reaction of sulfur trioxide and sulfur dichloride. This synthesis can be adapted to the laboratory by heating oleum to slowly distill the sulfur trioxide into a cooled fla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Aliphatic Substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :OH- + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organic photochemistry, photochemical reactions and organic redox reaction, redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels–Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SNi Reaction Mechanism
In chemistry, Si (substitution nucleophilic internal) refers to a specific, regio-selective but not often encountered reaction mechanism for nucleophilic aliphatic substitution. The name was introduced by Cowdrey et al. in 1937 to label nucleophilic reactions which occur with retention of configuration, but later was employed to describe various reactions that proceed with a similar mechanism. A typical representative organic reaction displaying this mechanism is the chlorination of alcohols with thionyl chloride, or the decomposition of alkyl chloroformates, the main feature is retention of stereochemical configuration. Some examples for this reaction were reported by Edward S. Lewis and Charles E. Boozer in 1952. Mechanistic and kinetic studies were reported few years later by various researchers. Thionyl chloride first reacts with the alcohol to form an alkyl chloro sulfite, actually forming an intimate ion pair. The second step is the loss of a sulfur dioxide molecule an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
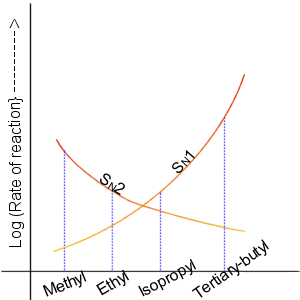
SN2 Reaction
The bimolecular nucleophilic substitution (SN2) is a type of reaction mechanism that is common in organic chemistry. In the SN2 reaction, a strong nucleophile forms a new bond to an sp3-hybridised carbon atom via a backside attack, all while the leaving group detaches from the reaction center in a concerted (i.e. simultaneous) fashion. The name SN2 refers to the Hughes-Ingold symbol of the mechanism: "SN" indicates that the reaction is a nucleophilic substitution, and "2" that it proceeds via a bimolecular mechanism, which means both the reacting species are involved in the rate-determining step. What distinguishes SN2 from the other major type of nucleophilic substitution, the SN1 reaction, is that the displacement of the leaving group, which is the rate-determining step, is separate from the nucleophilic attack in SN1. The SN2 reaction can be considered as an organic-chemistry analogue of the associative substitution from the field of inorganic chemistry. Reaction mech ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intimate Ion Pair
In chemistry, the intimate ion pair concept, introduced by Saul Winstein, describes the interactions between a cation, anion and surrounding solvent molecules. In ordinary aqueous solutions of inorganic salts, an ion is completely solvated and shielded from the counterion. In less polar solvents, two ions can still be connected to some extent. In a ''tight'', ''intimate'', or ''contact'' ion pair, there are no solvent molecules between the two ions. When solvation increases, ionic bonding decreases and a ''loose'' or ''solvent-shared'' ion pair results. The ion pair concept explains stereochemistry in solvolysis. The concept of intimate ion pairs is used to explain the slight tendency for inversion of stereochemistry during an S1 reaction. It is proposed that solvent or other ions in solution may assist in the removal of a leaving group to form a carbocation which reacts in an S1 fashion; similarly, the leaving group may associate loosely with the cationic intermediate. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Mustard
Mustard gas or sulfur mustard are names commonly used for the organosulfur chemical compound bis(2-chloroethyl) sulfide, which has the chemical structure S(CH2CH2Cl)2, as well as other species. In the wider sense, compounds with the substituents are known as ''sulfur mustards'' or '' nitrogen mustards'', respectively, where X = Cl or Br. Such compounds are potent alkylating agents, making mustard gas acutely and severely toxic. Mustard gas is a carcinogen. There is no preventative agent against mustard gas, with protection depending entirely on skin and airways protection, and no antidote exists for mustard poisoning. Also known as mustard agents, this family of compounds comprises infamous cytotoxins and blister agents with a long history of use as chemical weapons. The name ''mustard gas'' is technically incorrect; the substances, when dispersed, are often not gases but a fine mist of liquid droplets that can be readily absorbed through the skin and by inhalation. The ski ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Acyl Substitution
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group () or hydrogen in the case of formyl group (). In organic chemistry, the acyl group (IUPAC name alkanoyl if the organyl group is alkyl) is usually derived from a carboxylic acid, in which case it has the formula , where R represents an organyl group or hydrogen. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids and phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond. Reactivity trends There are five main types of acyl derivatives. Acid halides are the most reactive towards nucleophiles, followed by anhydrides, esters, and amides. Carboxylate ions are esse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged Atomic nucleus, atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as Alcohol (chemistry), alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. The difference between the two is, that basicity is a thermodynamic property (i.e. relates to an equilibrium state), but nucleop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
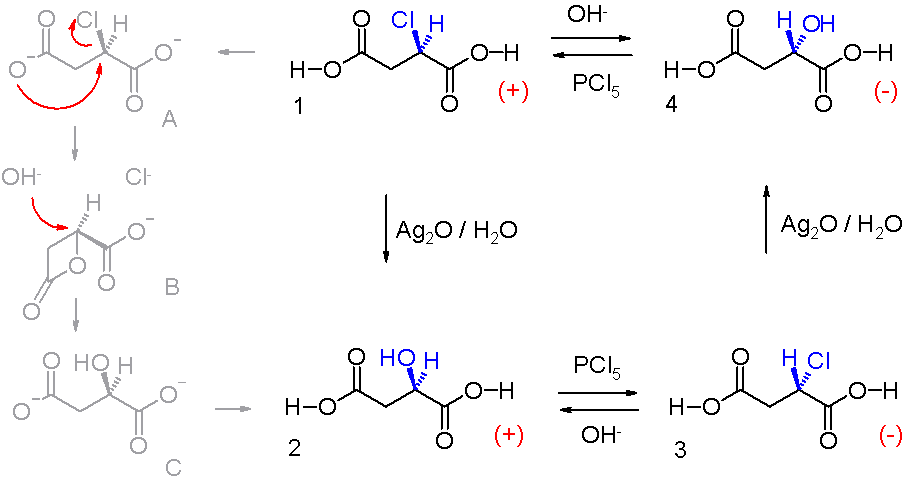
Walden Inversion
Walden inversion is the inversion of a stereogenic center in a chiral molecule in a chemical reaction. Since a molecule can form two enantiomers around a stereogenic center, the Walden inversion converts the configuration of the molecule from one enantiomeric form to the other. For example, in an SN2 reaction, Walden inversion occurs at a tetrahedral carbon atom. It can be visualized by imagining an umbrella turned inside-out in a gale. In the Walden inversion, the backside attack by the nucleophile in an SN2 reaction gives rise to a product whose configuration is opposite to the reactant. Therefore, during SN2 reaction, 100% inversion of product takes place. This is known as Walden inversion. It was first observed by chemist Paul Walden in 1896. He was able to convert one enantiomer of a chemical compound into the other enantiomer and back again in a so-called Walden cycle which went like this: (+)- chlorosuccinic acid (1 in the illustration) was converted to (+)-malic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |