Nucleophilic aliphatic substitution on:
[Wikipedia]
[Google]
[Amazon]
In OH- + R-Br -> R-OH + Br-
Nucleophilic substitution reactions are common in
 In 1935,
In 1935,
 The
The
CH3CH=CH-CH2-Cl -> CH3CH=CH-CH2-OH + CH3CH(OH)-CH=CH2
Another mechanism od Sn reactions is ONSH (oxidative nucleophilic substitution of hydrogen). One of the best-studied examples of the ONSH mechanism is the synthesis of substituted nitroindoles.
This reaction typically involves selected ketones capable of forming enolates in the presence of a strong base such as
SciTecLibrary
/ref> In
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, a nucleophilic substitution (SN) is a class of chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
s in which an electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
-rich chemical species
Chemical species are a specific form of chemical substance or chemically identical molecular entities that have the same molecular energy level at a specified timescale. These entities are classified through bonding types and relative abundance of ...
(known as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
) replaces a functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
within another electron-deficient molecule (known as the electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
). The molecule that contains the electrophile and the leaving functional group is called the substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (aquatic environment), the earthy material that exi ...
.
The most general form of the reaction may be given as the following:
:
The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged.
An example of nucleophilic substitution is the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of an alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retard ...
, R-Br under basic conditions, where the attacking nucleophile is hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
() and the leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
is bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retard ...
().
:organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
. Nucleophiles often attack a saturated aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated (in which all ...
carbon. Less often, they may attack an aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
or unsaturated carbon.
Saturated carbon centres
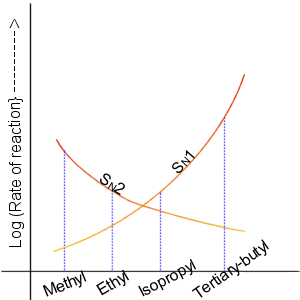
SN1 and SN2 reactions
 In 1935,
In 1935, Edward D. Hughes
Edward David Hughes (June 18, 1906June 30, 1963) was a British organic chemistry, organic chemist. He was a professor first at University College, Bangor and then at University College London, University College in London, eventually rising to the ...
and Sir Christopher Ingold studied nucleophilic substitution reactions of alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
s and related compounds. They proposed that there were two main mechanisms at work, both of them competing with each other. The two main mechanisms were the SN1 reaction and the SN2 reaction, where ''S'' stands for substitution, ''N'' stands for nucleophilic, and the number represents the kinetic order of the reaction.
In the SN2 reaction, the addition of the nucleophile and the elimination of leaving group take place simultaneously (i.e. a concerted reaction
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend not ...
). SN2 occurs when the central carbon atom is easily accessible to the nucleophile.
In SN2 reactions, there are a few conditions that affect the rate of the reaction. First of all, the 2 in SN2 implies that there are two concentrations of substances that affect the rate of reaction: substrate (Sub) and nucleophile. The rate equation for this reaction would be Rate=k ubNuc]. For a SN2 reaction, an Protic_solvent, aprotic solvent is best, such as acetone, DMF, or DMSO. Aprotic solvents do not add protons (H+ ions) into solution; if protons were present in SN2 reactions, they would react with the nucleophile and severely limit the reaction rate. Since this reaction occurs in one step, steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformational isomerism, co ...
drive the reaction speed. In the intermediate step, the nucleophile is 185 degrees from the leaving group and the stereochemistry is inverted as the nucleophile bonds to make the product. Also, because the intermediate is partially bonded to the nucleophile and leaving group, there is no time for the substrate to rearrange itself: the nucleophile will bond to the same carbon that the leaving group was attached to. A final factor that affects reaction rate is nucleophilicity; the nucleophile must attack an atom other than a hydrogen.
By contrast the SN1 reaction involves two steps. SN1 reactions tend to be important when the central carbon atom of the substrate is surrounded by bulky groups, both because such groups interfere sterically with the SN2 reaction (discussed above) and because a highly substituted carbon forms a stable carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
.
Like SN2 reactions, there are quite a few factors that affect the reaction rate of SN1 reactions. Instead of having two concentrations that affect the reaction rate, there is only one, substrate. The rate equation for this would be Rate=k ub Since the rate of a reaction is only determined by its slowest step, the rate at which the leaving group "leaves" determines the speed of the reaction. This means that the better the leaving group, the faster the reaction rate. A general rule for what makes a good leaving group is the weaker the conjugate base, the better the leaving group. In this case, halogens are going to be the best leaving groups, while compounds such as amines, hydrogen, and alkanes are going to be quite poor leaving groups. As SN2 reactions were affected by sterics, SN1 reactions are determined by bulky groups attached to the carbocation. Since there is an intermediate that actually contains a positive charge, bulky groups attached are going to help stabilize the charge on the carbocation through resonance and distribution of charge. In this case, tertiary carbocation will react faster than a secondary which will react much faster than a primary. It is also due to this carbocation intermediate that the product does not have to have inversion. The nucleophile can attack from the top or the bottom and therefore create a racemic product. It is important to use a protic solvent, water and alcohols, since an aprotic solvent could attack the intermediate and cause unwanted product. It does not matter if the hydrogens from the protic solvent react with the nucleophile since the nucleophile is not involved in the rate determining step.
Reactions
There are many reactions in organic chemistry involving this type of mechanism. Common examples include: *Organic reduction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions car ...
s with hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
s, for example
:: using (S2)
* Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
reactions such as
:: (S2) or
:: (S1)
* Williamson ether synthesis
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ...
:: (S2)
* The Wenker synthesis
The Wenker synthesis is an organic reaction converting a beta amino alcohol to an aziridine with the help of sulfuric acid. It is used industrially for the synthesis of aziridine itself.
The original Wenker synthesis of aziridine itself takes ...
, a ring-closing reaction of aminoalcohols.
* The Finkelstein reaction, a halide exchange reaction. Phosphorus nucleophiles appear in the Perkow reaction
The Perkow reaction is an organic reaction in which a trialkyl phosphite ester reacts with a haloketone to form a dialkyl vinyl group, vinyl phosphate and an alkyl halide.
In the related Michaelis–Arbuzov reaction the same reactants are known ...
and the Michaelis–Arbuzov reaction.
* The Kolbe nitrile synthesis
The Kolbe nitrile synthesis is a method for the preparation of alkyl nitriles by reaction of the corresponding organohalide, alkyl halide with a metal cyanide. A side product for this reaction is the formation of an isonitrile because the cyanide i ...
, the reaction of alkyl halides with cyanides.
Borderline mechanism
An example of a substitution reaction taking place by a so-called borderline mechanism as originally studied by Hughes and Ingold is the reaction of ''1-phenylethyl chloride'' withsodium methoxide
Sodium methoxide is the simplest sodium alkoxide. With the formula , it is a white solid, which is formed by the deprotonation of methanol. It is a widely used reagent in industry and the laboratory. It is also a dangerously caustic base.
...
in methanol.
:reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
is found to the sum of S1 and S2 components with 61% (3,5 M, 70 °C) taking place by the latter.
Other mechanisms
Besides S1 and S2, other mechanisms are known, although they are less common. The Si mechanism is observed in reactions ofthionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately Volatility (chemistry), volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a Halogenation, chlorinating reagen ...
with alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s, and it is similar to S1 except that the nucleophile is delivered from the same side as the leaving group.
Nucleophilic substitutions can be accompanied by an allylic rearrangement An allylic rearrangement or allylic shift is an organic reaction, organic chemical reaction in which reaction at a center Vicinal (chemistry), vicinal to a double bond causes the double bond to shift to an adjacent pair of atoms:
It is encountered ...
as seen in reactions such as the Ferrier rearrangement. This type of mechanism is called an S1' or S2' reaction (depending on the kinetics). With allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
ic halides or sulphonates, for example, the nucleophile may attack at the γ unsaturated carbon in place of the carbon bearing the leaving group. This may be seen in the reaction of 1-chloro-2-butene with sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
to give a mixture of 2-buten-1-ol and 1-buten-3-ol:
:Potassium tert-butoxide
Potassium ''tert''-butoxide (or potassium ''t''-butoxide) is a chemical compound with the formula CH3)3COKsub>''n'' (abbr. KOtBu). This colourless solid is a strong base (pKa of conjugate acid is 17 in H2O), which is useful in organic syn ...
, reacting with 3-Nitroaniline
3-Nitroaniline is an organic compound with the formula H2NC6H4NO2. A yellow solid, it is a derivative of aniline, carrying a nitro functional group in position 3. It is an isomer of 2-nitroaniline and 4-nitroaniline. It is used as a precursor t ...
as the second substrate.
The deprotonated enolate formed from the ketone acts as the nucleophile and attacks the 4- or 6-position of the 3-nitroaniline ring. This regioselectivity is governed by the strong electron-withdrawing effect of the nitro group, which activates the ortho and para positions (relative to itself) towards nucleophilic attack.
The Sn1CB mechanism appears in inorganic chemistry
Inorganic chemistry deals with chemical synthesis, synthesis and behavior of inorganic compound, inorganic and organometallic chemistry, organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subj ...
. Competing mechanisms exist.Unimolecular Nucleophilic Substitution does not Exist! / N.S.ImyanitovSciTecLibrary
/ref> In
organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
the nucleophilic abstraction reaction occurs with a nucleophilic substitution mechanism.
Unsaturated carbon centres
Nucleophilic substitution via the SN1 or SN2 mechanism does not generally occur with vinyl or aryl halides or related compounds. Under certain conditions nucleophilic substitutions may occur, via other mechanisms such as those described in thenucleophilic aromatic substitution
A nucleophilic aromatic substitution (SNAr) is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic c ...
article.
Substitution can occur at the carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
group, such as acyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
s and ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s.
References
External links
{{Authority control