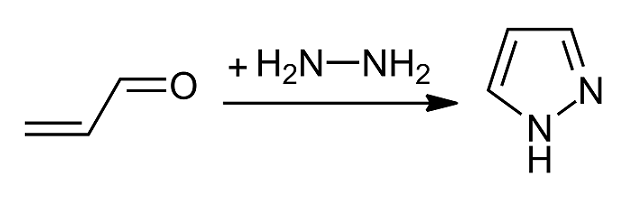|
S6821
S6821 is a food additive that acts as a potent and selective antagonist of the bitter taste The gustatory system or sense of taste is the sensory system that is partially responsible for the perception of taste. Taste is the perception stimulated when a substance in the mouth reacts chemically with taste receptor cells located on tas ... receptor TAS2R8. It has been approved as a food additive to block the bitter taste of certain active pharmaceutical ingredients, excipients, and nutraceuticals which primarily produce their bitter taste through binding to TAS2R8. See also * S9632 References Food additives Oxazoles Pyrazoles Hydantoins 3-Hydroxyphenyl compounds Ureas {{pharm-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TAS2R8
Taste receptor type 2 member 8 is a protein that in humans is encoded by the ''TAS2R8'' gene. Function This gene product belongs to the family of candidate taste receptors that are members of the G-protein-coupled receptor superfamily. These proteins are specifically expressed in the taste receptor cells of the tongue and palate epithelia. They are organized in the genome in clusters and are genetically linked to loci that influence bitter perception in mice and humans. In functional expression studies, they respond to bitter tastants. This gene maps to the taste receptor gene cluster on chromosome 12p13. Antagonists * S6821 See also * Taste receptor A taste receptor or tastant is a type of cellular receptor that facilitates the sensation of taste. When food or other substances enter the mouth, molecules interact with saliva and are bound to taste receptors in the oral cavity and other locat ... References Further reading * * * * * * * Human taste receptors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S9632
S9632 (FEMA 4774) is a food additive which acts as a positive allosteric modulator of the TAS1R2/TAS1R3 heterodimer that is the primary sweet taste receptor in humans. It is described as a flavour modifier, having little taste of its own but causing sweet flavours to taste sweeter and enhancing certain aspects of the flavour profile. It was approved for use in food in 2012 and is used in various countries including the United States, Japan, South Korea, and Mexico. See also * S6821 * Miraculin Miraculin is a taste modifier, a glycoprotein extracted from the fruit of '' Synsepalum dulcificum''. The berry, also known as the miracle fruit, was documented by explorer Chevalier des Marchais, who searched for many different fruits during ... References Food additives Taste modifiers Isopropylamino compounds Carboxamides Quinolines Carboxylic acids Amines {{pharm-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves ." '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, beta b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bitter Taste
The gustatory system or sense of taste is the sensory system that is partially responsible for the perception of taste. Taste is the perception stimulated when a substance in the mouth reacts chemically with taste receptor cells located on taste buds in the oral cavity, mostly on the tongue. Taste, along with the sense of smell and trigeminal nerve stimulation (registering texture, pain, and temperature), determines flavors of food and other substances. Humans have taste receptors on taste buds and other areas, including the upper surface of the tongue and the epiglottis. The gustatory cortex is responsible for the perception of taste. The tongue is covered with thousands of small bumps called papillae, which are visible to the naked eye. Within each papilla are hundreds of taste buds. The exceptions to this is the filiform papillae that do not contain taste buds. There are between 2000 and 5000Boron, W.F., E.L. Boulpaep. 2003. Medical Physiology. 1st ed. Elsevier Science U ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food Additives
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives, such as vinegar ( pickling), salt ( salting), smoke (smoking) and sugar (crystallization), have been used for centuries to preserve food. This allows for longer-lasting foods, such as bacon, sweets, and wines. With the advent of ultra-processed foods in the late 20th century, many additives having both natural and artificial origin were introduced. Food additives also include substances that may be introduced to food indirectly (called "indirect additives") in the manufacturing process through packaging, storage or transport. In Europe and internationally, many additives are designated with E numbers, while in the United States, additives in amounts deemed safe for human consumption are designated as GRAS. Identification To regulate these additives and inform consumers each additive is assigned a unique number called an "E number", which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazoles
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a p''K''a of 0.8, compared to 7 for imidazole. Preparation The classic synthetic route the Robinson–Gabriel synthesis by dehydration of 2-acylaminoketones: The Fischer oxazole synthesis from cyanohydrins and aldehydes is also widely used: Other methods are known including the reaction of α- haloketones and formamide and the Van Leusen reaction with aldehydes and TosMIC. Biosynthesis In biomolecules, oxazoles result from the cyclization and oxidation of serine or threonine nonribosomal peptides: : Oxazoles are not as abundant in biomolecules as the related thiazoles with oxygen replaced by a sulfur atom. Reactions With a pKa of 0.8 for the conjugate acid (oxazolium salts), oxazoles are far less ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazoles
Pyrazole is an organic compound with the formula . It is a heterocycle characterized as an azole with a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Pyrazole itself has few applications but many substituted pyrazoles are of commercial interest. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Properties Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). According to X-ray crystallography, the compound is planar. The two C-N distances are similar, both near 1.33 Å History The term pyrazole was given to this class of compounds by German Chemist Ludwig Knorr in 1883. In a classical method developed by German chemist Hans von Pechmann in 1898, pyrazole was synthesized from acetylene and diazomethane. Preparation Pyrazoles are syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydantoins
Hydantoin, or glycolylurea, is a heterocyclic organic compound with the formula CH2C(O)NHC(O)NH. It is a colorless solid that arises from the reaction of glycolic acid and urea. It is an oxidized derivative of imidazolidine. In a more general sense, hydantoins can refer to groups or a class of compounds with the same ring structure as the parent compound. For example, phenytoin (mentioned below) has two phenyl groups substituted onto the number 5 carbon in a hydantoin molecule. Synthesis Hydantoin was first isolated in 1861 by Adolf von Baeyer in the course of his study of uric acid. He obtained it by hydrogenation of allantoin, hence the name. : Friedrich Urech synthesized 5-methylhydantoin in 1873 from alanine sulfate and potassium cyanate in what is now known as the Urech hydantoin synthesis. The method is very similar to the modern route using alkyl and arylcyanates. The 5,5-dimethyl compound can also be obtained from acetone cyanohydrin (also discovered by Urech: see c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |