|
Rotterdam Convention
The Rotterdam Convention (formally, the Rotterdam Convention on the Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade) is a multilateral treaty to promote shared responsibilities in relation to importation of hazardous chemicals. The convention promotes open exchange of information and calls on exporters of hazardous chemicals to use proper labeling, include directions on safe handling, and inform purchasers of any known restrictions or bans. Signatory nations can decide whether to allow or ban the importation of chemicals listed in the treaty, and exporting countries are obliged to make sure that producers within their jurisdiction comply. In 2012, the Secretariats of the Basel and Stockholm conventions, as well as the UNEP-part of the Rotterdam Convention Secretariat, merged to a single Secretariat with a matrix structure serving the three conventions. The three conventions now hold back to back Conferences of the Parties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rotterdam Rules
The "Rotterdam Rules" (formally, the United Nations Convention on Contracts for the International Carriage of Goods Wholly or Partly by Sea) is a treaty proposing new international rules to revise the legal framework for maritime affreightment and carriage of goods by sea. The Rules primarily address the legal relationship between carriers and cargo-owners. The aim of the convention is to extend and modernize existing international rules and achieve uniformity of International trade law in the field of maritime carriage, updating or replacing many provisions in the Hague Rules, Hague-Visby Rules and Hamburg Rules. The convention establishes a comprehensive, uniform legal regime governing the rights and obligations of shippers, carriers and consignees under a contract for door-to-door shipments that involve international sea transport. Although the final text was greeted with much enthusiasm, a decade later, little has happened. As of December 2018, the rules are not yet in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlordane
Chlordane, or chlordan, is an organochlorine compound that was used as a pesticide. It is a white solid. In the United States, chlordane was used for termite-treatment of approximately 30 million homes until it was banned in 1988. Chlordane was banned 10 years earlier for food crops like corn and citrus, and on lawns and domestic gardens.Robert L. Metcalf "Insect Control" in ''Ullmann’s Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2002. Like other chlorinated cyclodiene insecticides, chlordane is classified as an organic pollutant hazardous for human health. It is resistant to degradation in the environment and in humans/animals and readily accumulates in lipids (fats) of humans and animals.Agency for Toxic Substances & Disease Registry (ATSDR). Toxic Substances Portal: Chlordane. Last updated September, 2010 nline Available at URL: http://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=62 Exposure to the compound has been linked to cancers, diabetes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexachlorobenzene
Hexachlorobenzene, or perchlorobenzene, is an organochloride with the molecular formula C6Cl6. It is a fungicide formerly used as a seed treatment, especially on wheat to control the fungal disease bunt. It has been banned globally under the Stockholm Convention on Persistent Organic Pollutants. Physical and chemical properties Hexachlorobenzene is a stable, white, crystalline chlorinated hydrocarbon. It is sparingly soluble in organic solvents such as benzene, diethyl ether and alcohol, but practically insoluble in water with no reaction. It reacts violently above 65 °C with dimethyl formamide and has a flash point of 468 °F. It is stable under normal temperatures and pressures. It is combustible but it does not ignite readily. When heated to decomposition, hexachlorobenzene emits highly toxic fumes of hydrochloric acid, other chlorinated compounds (such as phosgene), carbon monoxide, and carbon dioxide. Synthesis Hexachlorobenzene has been made on a laboratory scale since t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heptachlor
Heptachlor is an organochlorine compound that was used as an insecticide. Usually sold as a white or tan powder, heptachlor is one of the cyclodiene insecticides. In 1962, Rachel Carson's '' Silent Spring'' questioned the safety of heptachlor and other chlorinated insecticides. Due to its highly stable structure, heptachlor can persist in the environment for decades. In the United States, the Environmental Protection Agency has limited the sale of heptachlor products to the specific application of fire ant control in underground transformers. The amount that can be present in different foods is regulated.Robert L. Metcalf "Insect Control" in ''Ullmann’s Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2002. Synthesis Analogous to the synthesis of other cyclodienes, heptachlor is produced via the Diels-Alder reaction of hexachlorocyclopentadiene and cyclopentadiene. The resulting adduct is chlorinated followed by treatment with hydrogen chloride in nitromet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexachlorocyclohexane
Hexachlorocyclohexane (HCH), , is any of several polyhalogenated organic compounds consisting of a six-carbon ring with one chlorine and one hydrogen attached to each carbon. This structure has nine stereoisomers (eight diastereomers, one of which has two enantiomers), which differ by the stereochemistry of the individual chlorine substituents on the cyclohexane. It is sometimes erroneously called " benzene hexachloride" (BHC). They have been used as models for analyzing the effects of different geometric positions of the large atoms with dipolar bonds on the stability of the cyclohexane conformation. The isomers are poisonous, pesticidal, and persistent organic pollutants, to varying degrees. Hexachlorocyclohexane was dimerized to produce mirex, a banned pesticide. Common forms are: * ''alpha''-hexachlorocyclohexane, α-HCH, or α-BHC ( CAS RN: ), the optically active isomer * ''beta''-hexachlorocyclohexane, β-HCH, or β-BHC (CAS RN: ) * ''gamma''-hexachlorocyclohexane, γ- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroacetamide
Fluoroacetamide is an organic compound based on acetamide with one fluorine Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ... atom replacing hydrogen on the methyl group. it is a metabolic poison which disrupts the citric acid cycle and was used as a rodenticide.MATSUMURA F, O'BRIEN RD. A COMPARATIVE STUDY OF THE MODES OF ACTION OF FLUOROACETAMIDE AND FLUOROACETATE IN THE MOUSE AND AMERICAN COCKROACH. ''Biochem Pharmacol''. 1963 Oct;12:1201-5. See also * Sodium fluoroacetate References Rodenticides Acetamides Organofluorides Respiratory toxins {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Oxide
Ethylene oxide is an organic compound with the formula . It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of silver catalyst. The reactivity that is responsible for many of ethylene oxide's hazards also makes it useful. Although too dangerous for direct household use and generally unfamiliar to consumers, ethylene oxide is used for making many consumer products as well as non-consumer chemicals and intermediates. These products include detergents, thickeners, solvents, plastics, and various organic chemicals such as ethylene glycol, ethanolamines, simple an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Dichloride
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water (at a boiling point of ) and other chlorocarbons. History In 1794, physician Jan Rudolph Deiman, merchant Adriaan Paets van Troostwijk, chemist Anthoni Lauwerenburg, and botanist Nicolaas Bondt, under the name of Society of Dutch Chemists ( nl, Gezelschap der Hollandsche Scheikundigen), were the first to produce 1,2-dichloroethane from olefiant gas (oil-making gas, ethylene) and chlorine gas. Although the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endosulfan
Endosulfan is an off- patent organochlorine insecticide and acaricide that is being phased out globally. It became a highly controversial agrichemical due to its acute toxicity, potential for bioaccumulation, and role as an endocrine disruptor. Because of its threats to human health and the environment, a global ban on the manufacture and use of endosulfan was negotiated under the Stockholm Convention in April 2011. The ban has taken effect in mid-2012, with certain uses exempted for five additional years. More than 80 countries, including the European Union, Australia, New Zealand, several West African nations, the United States,Cone, MarlaEPA Bans Pesticide Found on Cucumbers, Zucchini, Green Beans and Other Vegetables.''The Daily Green.'' June 10, 2010. Brazil, and Canada had already banned it or announced phase-outs by the time the Stockholm Convention ban was agreed upon. It is still used extensively in India and China despite laws against its use. It is also used in a fe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-dibromoethane
1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula . Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly synthetic. It is a dense colorless liquid with a faint sweet odor, detectable at 10 ppm, is a widely used and sometimes-controversial fumigant. The combustion of 1,2-dibromoethane produces hydrogen bromide gas that is significantly corrosive. Preparation and use It is produced by the reaction of ethylene gas with bromine, in a classic halogen addition reaction: :CH=CH + Br → BrCH–CHBr Historically, 1,2-dibromoethane was used as a component in anti-knock additives in leaded fuels. It reacts with lead residues to generate volatile lead bromides, thereby preventing fouling of the engine with lead deposits. Pesticide It has been used as a pesticide in soil and on various crops. The applications were initiated after the forced retirement of 1,2- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
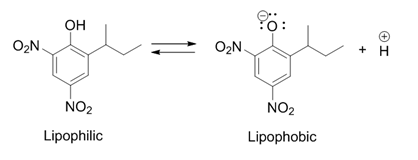
Dinoseb
Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a herbicide in the dinitrophenol family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union (EU) and the United States because of its toxicity. It also finds use as a polymerisation inhibitor, where it is often referred to as DNBP. It is used to prevent the thermally induced polymerisation of styrene and other unsaturated monomers when they are being purified by distillation. History In 1892, dinitro-''ortho''-cresol (2,4-dinitro-6-methylphenol), a chemical compound closely related to dinoseb, was discovered in Germany and first used as an insecticide. It was later also used as an herbicide and also fungicide after those characteristics were discovered. In 1945 the ''ortho''-methyl group was replaced by a ''sec''-butyl group, producing dinoseb. This compound had a superior contact and stomach activity on insects and mites. Dino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitro-ortho-cresol
Dinitro-''ortho''-cresol (DNOC) is an organic compound with the structural formula CH3C6H2(NO2)2OH. It is a yellow solid that is only slightly soluble in water. DNOC and some related derivatives have been used as herbicides. Preparation This compound is prepared by disulfonation of ''o''-cresol. The resulting disulfonate is then treated with nitric acid to give DNOC. A variety of related derivatives are known including those where the methyl group is replaced by ''sec''-butyl ( dinoseb), ''tert''-butyl ( dinoterb), and 1-methylheptyl ( dinocap). These are prepared by the direct nitration of the alkyphenols. Applications and safety This toxicant is an uncoupler, which means that it interferes with adenosine triphosphate (ATP) production. Symptoms of dinitro-''ortho''-cresol poisoning, due to ingestion or other forms of exposure, include confusion, headache, shortness of breath, and sweat Perspiration, also known as sweating, is the production of fluids secreted by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |