|
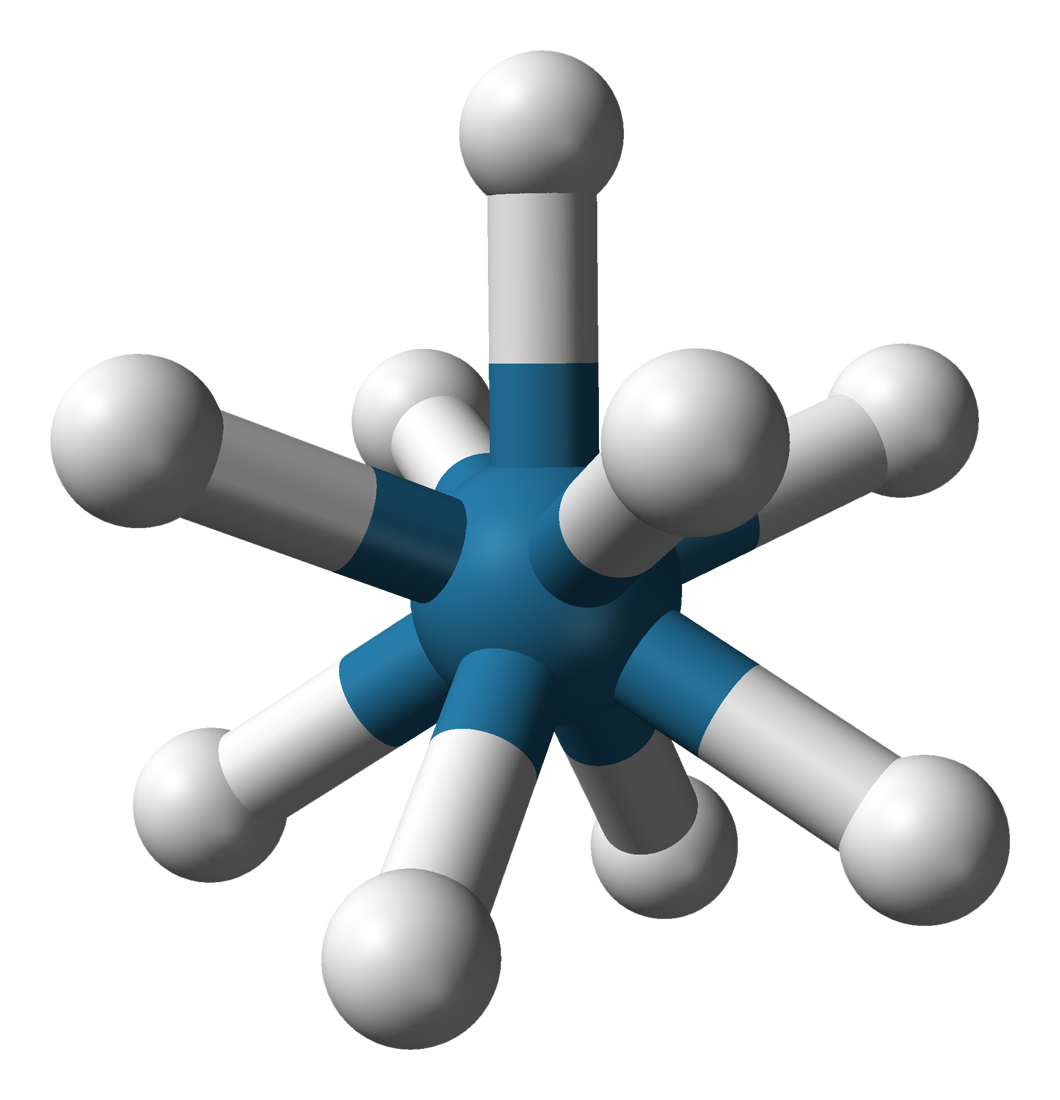
Rhenium(IV) Iodide
Rhenium tetraiodide is a binary chemical compound of rhenium and iodide with the chemical formula . Synthesis Rhenium tetraiodide can be obtained via the reduction of perrhenic acid with hydrogen iodide: :: Physical properties Rhenium tetraiodide forms black solid substance which is soluble in acetone and ether In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R†.... Hydrolyzed by water, hygroscopic. Chemical properties Rhenium tetraiodide is hydrolyzed by water: :: Rhenium tetraiodide decomposes when heated: :: References Rhenium(IV) compounds Iodides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iridium Tetraiodide
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density of as defined by experimental X-ray crystallography. 191Ir and 193Ir are the only two naturally occurring isotopes of iridium, as well as the only stable isotopes; the latter is the more abundant. It is one of the most corrosion-resistant metals, even at temperatures as high as . Iridium was discovered in 1803 in the acid-insoluble residues of platinum ores by the English chemist Smithson Tennant. The name ''iridium'', derived from the Greek word ''iris'' (rainbow), refers to the various colors of its compounds. Iridium is one of the rarest elements in Earth's crust, with an estimated annual production of only in 2023. The dominant uses of iridium are the metal itself and its alloys, as in high-performance spark plugs, crucibles for rec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. It has one of the highest melting and boiling points of any element. It resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. It shows in its compounds a wide variety of oxidation states ranging from −1 to +7. Rhenium was originally discovered in 1908 by Masataka Ogawa, but he mistakenly assigned it as element 43 (now known as technetium) rather than element 75 and named it ''nipponium''. It was rediscovered in 1925 by Walter Noddack, Ida Tacke and Otto Berg, who gave it its present name. It was named after the river Rhine in Europe, from which the earliest samples had been obtained and worked co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is not. The l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature (journal)
''Nature'' is a British weekly scientific journal founded and based in London, England. As a multidisciplinary publication, ''Nature'' features Peer review, peer-reviewed research from a variety of academic disciplines, mainly in science and technology. It has core editorial offices across the United States, continental Europe, and Asia under the international scientific publishing company Springer Nature. ''Nature'' was one of the world's most cited scientific journals by the Science Edition of the 2022 ''Journal Citation Reports'' (with an ascribed impact factor of 50.5), making it one of the world's most-read and most prestigious academic journals. , it claimed an online readership of about three million unique readers per month. Founded in the autumn of 1869, ''Nature'' was first circulated by Norman Lockyer and Alexander MacMillan (publisher), Alexander MacMillan as a public forum for scientific innovations. The mid-20th century facilitated an editorial expansion for the j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perrhenic Acid
Perrhenic acid is the chemical compound with the formula . It is obtained by evaporating aqueous solutions of . Conventionally, perrhenic acid is considered to have the formula , and a species of this formula forms when rhenium(VII) oxide sublimes in the presence of water or steam. When a solution of is kept for a period of months, it breaks down and crystals of are formed, which contain tetrahedral . For most purposes, perrhenic acid and rhenium(VII) oxide are used interchangeably. Rhenium can be dissolved in nitric or concentrated sulfuric acid to produce perrhenic acid. Properties The structure of solid perrhenic acid is . This species is a rare example of a metal oxide coordinated to water; most often metal–oxo–aquo species are unstable with respect to their corresponding hydroxides: : The two rhenium atoms have different bonding geometries, with one being tetrahedral and the other octahedral, and with the water ligands coordinated to the latter. Gaseous perrhenic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Iodide
Hydrogen iodide (HI) is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent. Properties of hydrogen iodide HI is a colorless gas that reacts with oxygen to give water and iodine. With moist air, HI gives a mist (or fumes) of hydroiodic acid. It is exceptionally soluble in water, giving hydroiodic acid. One liter of water will dissolve 425 liters of HI gas, the most concentrated solution having only four water molecules per molecule of HI. Hydroiodic acid Hydroiodic acid is an aqueous solution of hydrogen iodide. Commercial "concentrated" hydroiodic acid usually contains 48–57% HI by mass. The solut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elsevier
Elsevier ( ) is a Dutch academic publishing company specializing in scientific, technical, and medical content. Its products include journals such as ''The Lancet'', ''Cell (journal), Cell'', the ScienceDirect collection of electronic journals, ''Trends (journals), Trends'', the ''Current Opinion (Elsevier), Current Opinion'' series, the online citation database Scopus, the SciVal tool for measuring research performance, the ClinicalKey search engine for clinicians, and the ClinicalPath evidence-based cancer care service. Elsevier's products and services include digital tools for Data management platform, data management, instruction, research analytics, and assessment. Elsevier is part of the RELX Group, known until 2015 as Reed Elsevier, a publicly traded company. According to RELX reports, in 2022 Elsevier published more than 600,000 articles annually in over 2,800 journals. As of 2018, its archives contained over 17 million documents and 40,000 Ebook, e-books, with over one b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a characteristic pungent odor. Acetone is miscibility, miscible with properties of water, water and serves as an important organic solvent in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and for production of methyl methacrylate and bisphenol A, which are precursors to widely used plastics.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in organic chemistry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of ox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium(IV) Compounds
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. It has one of the highest melting and boiling points of any element. It resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. It shows in its compounds a wide variety of oxidation states ranging from −1 to +7. Rhenium was originally discovered in 1908 by Masataka Ogawa, but he mistakenly assigned it as element 43 (now known as technetium) rather than element 75 and named it ''nipponium''. It was rediscovered in 1925 by Walter Noddack, Ida Tacke and Otto Berg, who gave it its present name. It was named after the river Rhine in Europe, from which the earliest samples had been obtained and worked commer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |